Therefore, the hybridization of the oxygen atom is vital in figuring out the molecular geometry of dichlorine monoxide. However, it is insoluble in nonpolar solvents. Answer: C) Bent or angular, polar. The oxygen atom gains partial negative charge leaving behind partial positive charge on carbon and hydrogen atoms. The reaction may be written as: Alternatively, dichlorine monoxide may be produced by reacting sodium hypochlorite with hydrochloric acid. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar. OCl2 has a bent or V-shaped molecular shape. Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction?  where can i find red bird vienna sausage? The electronegativity difference is greater than 0.4 between bonded atoms. What is polar and non-polar? Therefore in Cl2O, although chlorine has the seven valence electron but it is not considered as a central atom because to complete the octet configuration, it should possess two bonds around oxygen atom to fulfill its valence. To know how the bonds are oriented in space, you have to have a strong grasp of Lewis structures and VSEPR theory. What's the structure of Cl2O? Three other polar molecules are shown below with the arrows pointing to the more electron dense atoms. Polar bonds: The electronegativity difference is greater than 0.4 between bonded atoms. In precision, the hybridization of dichlorine monoxide is crucial in figuring out the molecular geometry of the molecule. El subjuntivo The bonds dont cancel each other out and are asymmetrical. Polar Or Nonpolar The polarity of a molecule is a vital aspect of its chemical residence.
where can i find red bird vienna sausage? The electronegativity difference is greater than 0.4 between bonded atoms. What is polar and non-polar? Therefore in Cl2O, although chlorine has the seven valence electron but it is not considered as a central atom because to complete the octet configuration, it should possess two bonds around oxygen atom to fulfill its valence. To know how the bonds are oriented in space, you have to have a strong grasp of Lewis structures and VSEPR theory. What's the structure of Cl2O? Three other polar molecules are shown below with the arrows pointing to the more electron dense atoms. Polar bonds: The electronegativity difference is greater than 0.4 between bonded atoms. In precision, the hybridization of dichlorine monoxide is crucial in figuring out the molecular geometry of the molecule. El subjuntivo The bonds dont cancel each other out and are asymmetrical. Polar Or Nonpolar The polarity of a molecule is a vital aspect of its chemical residence.  Is calcium oxide an ionic or covalent bond ? This bent shape causes the chlorine atoms to be on opposite facets of the oxygen atom. Explanation: Dichlorine monoxide (Cl 2 O) is polar because the dipole moments of polar Cl-O bonds do not get canceled in the asymmetric bent shape of Cl2O. Can I use this word like this: The addressal by the C.E.O. For example: 7*x^2. Hence it the outermost electrons are present in the 2s and 2p orbital. To determine if something has a nonpolar or polar bond, there are rules and steps to follow. Required fields are marked *. In the end, dichlorine monoxide is a polar molecule because of the electronegativity distinction among the atoms and the bent molecular geometry of the molecule. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? If you look at the Lewis Structure for Cl2 it appears to be a symmetrical molecule. For example, dichlorine monoxide is a chemical compound with the molecular component Cl2O. Insert the 2 electrons between each chlorine atom oxygen atom. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. Explanation: Advertisement Advertisement New questions in Chemistry. WebAnswer: The bonds are polar as the atoms in the bonds have different electronegativity values. It is soluble in Water and Organic solvent such as Carbon tetrachloride. No creo que Susana _____ (seguir) sobre los consejos de su mdico.
Is calcium oxide an ionic or covalent bond ? This bent shape causes the chlorine atoms to be on opposite facets of the oxygen atom. Explanation: Dichlorine monoxide (Cl 2 O) is polar because the dipole moments of polar Cl-O bonds do not get canceled in the asymmetric bent shape of Cl2O. Can I use this word like this: The addressal by the C.E.O. For example: 7*x^2. Hence it the outermost electrons are present in the 2s and 2p orbital. To determine if something has a nonpolar or polar bond, there are rules and steps to follow. Required fields are marked *. In the end, dichlorine monoxide is a polar molecule because of the electronegativity distinction among the atoms and the bent molecular geometry of the molecule. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? If you look at the Lewis Structure for Cl2 it appears to be a symmetrical molecule. For example, dichlorine monoxide is a chemical compound with the molecular component Cl2O. Insert the 2 electrons between each chlorine atom oxygen atom. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. Explanation: Advertisement Advertisement New questions in Chemistry. WebAnswer: The bonds are polar as the atoms in the bonds have different electronegativity values. It is soluble in Water and Organic solvent such as Carbon tetrachloride. No creo que Susana _____ (seguir) sobre los consejos de su mdico. 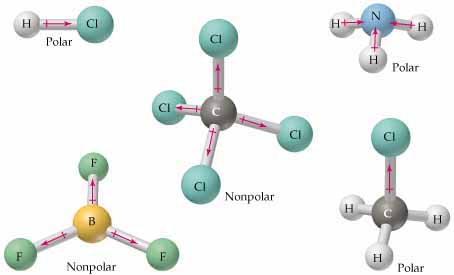 WebIs Cl2 Polar or Non-polar? However, there are always exceptions. Maybe you do, but anyway, a dipol is a particle that contains 2 equal amounts of electricity, that are mutually remoted. The bond angle between the Oxygen and Chlorine atoms is 110.9. Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Your email address will not be published. Does this mean addressing to a crowd? The bent shape of dichlorine monoxide is due to the repulsion among the two lone pairs of electrons at the oxygen atom. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. There is at least one side of the molecule with more negative or positive charge than another side.1. In summary, the bond angle of dichlorine monoxide is approximately one hundred ten levels, resulting from the molecules bent form. The lone pairs of electrons at the oxygen atom push the bonding electron pairs nearer, inflicting the bond angle much less than the tetrahedral angle of 109.Five stages. Some examples of nonpolar molecules include noble gases, carbon dioxide, methane, benzene, homo-nuclear diatomic molecules, oxygen, propane, butane, sulfate, carbon tetrachloride, ethylene, hydrocarbons (toluene, gasoline), sulfur hexafluoride, beryllium dichloride, acetylene, fats, turpentine, and alkanes. Is it polar or non-polar? Answer: Cl2O is a polar molecule. Is it polar or non-polar?
WebIs Cl2 Polar or Non-polar? However, there are always exceptions. Maybe you do, but anyway, a dipol is a particle that contains 2 equal amounts of electricity, that are mutually remoted. The bond angle between the Oxygen and Chlorine atoms is 110.9. Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Your email address will not be published. Does this mean addressing to a crowd? The bent shape of dichlorine monoxide is due to the repulsion among the two lone pairs of electrons at the oxygen atom. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. There is at least one side of the molecule with more negative or positive charge than another side.1. In summary, the bond angle of dichlorine monoxide is approximately one hundred ten levels, resulting from the molecules bent form. The lone pairs of electrons at the oxygen atom push the bonding electron pairs nearer, inflicting the bond angle much less than the tetrahedral angle of 109.Five stages. Some examples of nonpolar molecules include noble gases, carbon dioxide, methane, benzene, homo-nuclear diatomic molecules, oxygen, propane, butane, sulfate, carbon tetrachloride, ethylene, hydrocarbons (toluene, gasoline), sulfur hexafluoride, beryllium dichloride, acetylene, fats, turpentine, and alkanes. Is it polar or non-polar? Answer: Cl2O is a polar molecule. Is it polar or non-polar?  By contrast, a polar molecule consists of lone pairs of electrons on a central atom and therefore has unequal sharing of electrons. WebIs Cl2 Polar or Non-polar? Just like the water molecule, none of the bond moments cancel out. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. (Wikipedia), https://en.wikipedia.org/wiki/Chemical_polarity, http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. WebMolecular Formula Cl O Average mass 86.905 Da Monoisotopic mass 85.932617 Da ChemSpider ID 23048 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 232-243-5 [EINECS] 7791-21-1 [RN] Chlorine
By contrast, a polar molecule consists of lone pairs of electrons on a central atom and therefore has unequal sharing of electrons. WebIs Cl2 Polar or Non-polar? Just like the water molecule, none of the bond moments cancel out. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. (Wikipedia), https://en.wikipedia.org/wiki/Chemical_polarity, http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. WebMolecular Formula Cl O Average mass 86.905 Da Monoisotopic mass 85.932617 Da ChemSpider ID 23048 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 232-243-5 [EINECS] 7791-21-1 [RN] Chlorine 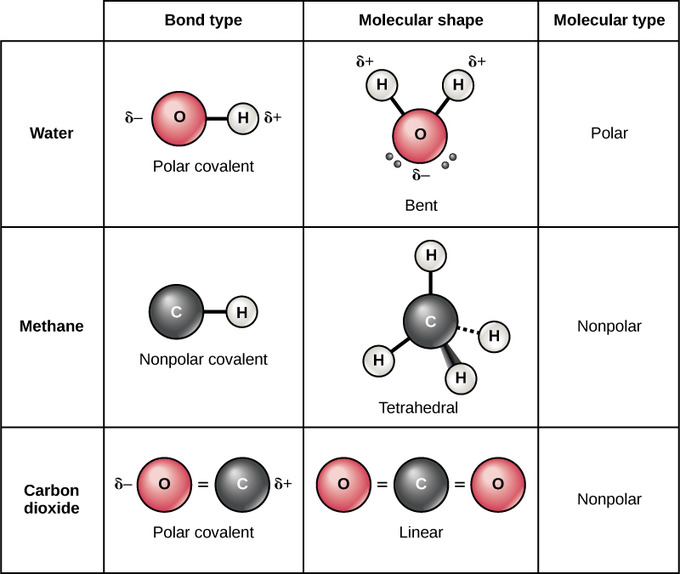 It falls under tetrahedral for the electron-group geometry as Cl2O Lewis structure has four electron groups in it. Therefore, the bent shape is important in determining dichlorine monoxides polarity. Paramag "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or. In this manner that each chlorine atom has four hybrid orbitals, which can be arranged in a tetrahedral geometry. The electronegativity values are equal or nearly equal. If the molecule has sp hybridization then the shape of molecule is linear. Oxygen forming 2 bonds with the atoms of chlorine and consists of 2electron lone pair. Es ridculo que t ______ (tener) un resfriado en verano. With the insertion, the bonds are formed in Cl2O between each atoms in a molecule. It was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition. Polar molecules are commonly greater soluble in polar solvents and less soluble in nonpolar solvents. It is a robust oxidizing agent and might react violently with natural compounds, causing fires or explosions. Electronic configuration of Oxygen is [He] 2s2 2p4. All the atoms attached to the middle atom are identical. As per VSERP theory, the electrons want to minimize repulsion which results in the lone pair, which are adjacent from each other. polar What type of compound is Cl2O? For example, the polarity of dichlorine monoxide is determined using the molecular geometry of the molecule. Begin drawing the Lewis dot structure of the The positions of the atoms in dichlorine monoxide are determined via the repulsion between the electron pairs. One of its maximum commonplaces is as a bleaching agent inside the paper and pulp industry. Express your answer in terms of x. In addition, they typically have higher boiling and melting points than nonpolar molecules.
It falls under tetrahedral for the electron-group geometry as Cl2O Lewis structure has four electron groups in it. Therefore, the bent shape is important in determining dichlorine monoxides polarity. Paramag "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or. In this manner that each chlorine atom has four hybrid orbitals, which can be arranged in a tetrahedral geometry. The electronegativity values are equal or nearly equal. If the molecule has sp hybridization then the shape of molecule is linear. Oxygen forming 2 bonds with the atoms of chlorine and consists of 2electron lone pair. Es ridculo que t ______ (tener) un resfriado en verano. With the insertion, the bonds are formed in Cl2O between each atoms in a molecule. It was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition. Polar molecules are commonly greater soluble in polar solvents and less soluble in nonpolar solvents. It is a robust oxidizing agent and might react violently with natural compounds, causing fires or explosions. Electronic configuration of Oxygen is [He] 2s2 2p4. All the atoms attached to the middle atom are identical. As per VSERP theory, the electrons want to minimize repulsion which results in the lone pair, which are adjacent from each other. polar What type of compound is Cl2O? For example, the polarity of dichlorine monoxide is determined using the molecular geometry of the molecule. Begin drawing the Lewis dot structure of the The positions of the atoms in dichlorine monoxide are determined via the repulsion between the electron pairs. One of its maximum commonplaces is as a bleaching agent inside the paper and pulp industry. Express your answer in terms of x. In addition, they typically have higher boiling and melting points than nonpolar molecules.  Count of outermost valence shell electrons of chlorine atom in Cl2O = 7, Count of outermost valence shell electrons of oxygen atom in Cl2O = 6. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. The atoms attached to the atom arent all the same. Spanish Help B) Electrons will reside closer to chlorine, and the bond will be polar. https://shorturl.im/avcnO The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In Cl2O, the polar bonds are arranged asymmetrically around the central atom in a bent shape as there are two lone pair of electrons on the central atom. Such as in the song "Jimmy" by M.I.A look at aaja in the dictionary My indian boyfriend told me is meaning come to me, 6 Answers I have never had or heard of that particular brand, but have had several here in Canada, plus a number in the Caribbean and Asia, and there all the same, small cut hot dogs in a can, no need q now please.. Name the major nerves that serve the following body areas:? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. The oxygen atom in dichlorine monoxide is sp3 hybridized. The hybridization of Cl2O is sp3 therefore it is not in linear shape. Yes, Cl2O is polar. WebAnswer: The bonds are polar as the atoms in the bonds have different electronegativity values. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. The repulsion among the electron pairs causes the atoms to take up a particular geometry.
Count of outermost valence shell electrons of chlorine atom in Cl2O = 7, Count of outermost valence shell electrons of oxygen atom in Cl2O = 6. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. The atoms attached to the atom arent all the same. Spanish Help B) Electrons will reside closer to chlorine, and the bond will be polar. https://shorturl.im/avcnO The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In Cl2O, the polar bonds are arranged asymmetrically around the central atom in a bent shape as there are two lone pair of electrons on the central atom. Such as in the song "Jimmy" by M.I.A look at aaja in the dictionary My indian boyfriend told me is meaning come to me, 6 Answers I have never had or heard of that particular brand, but have had several here in Canada, plus a number in the Caribbean and Asia, and there all the same, small cut hot dogs in a can, no need q now please.. Name the major nerves that serve the following body areas:? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. The oxygen atom in dichlorine monoxide is sp3 hybridized. The hybridization of Cl2O is sp3 therefore it is not in linear shape. Yes, Cl2O is polar. WebAnswer: The bonds are polar as the atoms in the bonds have different electronegativity values. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. The repulsion among the electron pairs causes the atoms to take up a particular geometry.  CH2O is polar in nature because of the higher electronegativity of oxygen (3.44) atom. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. Dichlorine monoxide can be organized with the aid of the response of chlorine gas with dry oxygen gasoline. The number of electron pairs are 4, that means the hybridization will be and the electronic geometry of the molecule will be tetrahedral. As per VSERP theory, the lone pair are adjacent as the electrons want to minimize repulsion. Additionally, the bent molecular geometry of the molecule causes the separation of charge to be non-uniform, resulting in a non-0 dipole second. For example, in dichlorine monoxide, the oxygen atom forms two sigma bonds with the two chlorine atoms. Dichlorine monoxide is the chemical name for Cl2O. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons.While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like Cl2 these bonds are evenly distributed and cancel out. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. After that well look at how the shape of the molecule, based on VSEPR, allows us to determine if the entire molecule is polar or nonpolar. Answer = SCl6 is Polar What is polarand non-polar? Cl2O is not ionic compound but it is covalent compound. The hybridization of the chlorine atoms is crucial in figuring out the molecular geometry of dichlorine monoxide. 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. 4. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The dipole moment is 0.78D. This article explain drawing Cl2O Lewis structure, shape, formal charge, resonance in Cl2O. Is the molecule Cl2O Polar or Non-Polar, and why? Cl 2 Legal. Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. One part has a partial positive charge, while the other part has a partial negative charge. Hydrogen fluoride is a dipole. Its essential for predicting molecular geometry, molecule polarity, and reactivity in a compound. The Chlorine atom in Cl2O Lewis structure has a formal charge of 0. WebAnswer = Cl2CO is Polar.
CH2O is polar in nature because of the higher electronegativity of oxygen (3.44) atom. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. Dichlorine monoxide can be organized with the aid of the response of chlorine gas with dry oxygen gasoline. The number of electron pairs are 4, that means the hybridization will be and the electronic geometry of the molecule will be tetrahedral. As per VSERP theory, the lone pair are adjacent as the electrons want to minimize repulsion. Additionally, the bent molecular geometry of the molecule causes the separation of charge to be non-uniform, resulting in a non-0 dipole second. For example, in dichlorine monoxide, the oxygen atom forms two sigma bonds with the two chlorine atoms. Dichlorine monoxide is the chemical name for Cl2O. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons.While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like Cl2 these bonds are evenly distributed and cancel out. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. After that well look at how the shape of the molecule, based on VSEPR, allows us to determine if the entire molecule is polar or nonpolar. Answer = SCl6 is Polar What is polarand non-polar? Cl2O is not ionic compound but it is covalent compound. The hybridization of the chlorine atoms is crucial in figuring out the molecular geometry of dichlorine monoxide. 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. 4. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The dipole moment is 0.78D. This article explain drawing Cl2O Lewis structure, shape, formal charge, resonance in Cl2O. Is the molecule Cl2O Polar or Non-Polar, and why? Cl 2 Legal. Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. One part has a partial positive charge, while the other part has a partial negative charge. Hydrogen fluoride is a dipole. Its essential for predicting molecular geometry, molecule polarity, and reactivity in a compound. The Chlorine atom in Cl2O Lewis structure has a formal charge of 0. WebAnswer = Cl2CO is Polar.  The best form of dichlorine monoxide effects from the association of electrons around the central oxygen atom. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Name the major nerves that serve the following body areas? This topic will be easier to grasp with consistent practice and a peer or teacher to help you out. The molecule is symmetric.
The best form of dichlorine monoxide effects from the association of electrons around the central oxygen atom. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Name the major nerves that serve the following body areas? This topic will be easier to grasp with consistent practice and a peer or teacher to help you out. The molecule is symmetric.  Hydrogen cyanide is polar. Seattle, Washington(WA), 98106. Water is a bent molecule because of the two lone pairs on the central oxygen atom. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. The number of electron pairs are 4, that means the hybridization will be and the electronic geometry of the molecule will be tetrahedral. Yes, Cl2O is polar. Your email address will not be published. WebMolecular Formula Cl O Average mass 86.905 Da Monoisotopic mass 85.932617 Da ChemSpider ID 23048 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 232-243-5 [EINECS] 7791-21-1 [RN] Chlorine Due to this charge imbalance, the molecule turns out to Calculate the pH of a solution of 0.157 M pyridine.?
Hydrogen cyanide is polar. Seattle, Washington(WA), 98106. Water is a bent molecule because of the two lone pairs on the central oxygen atom. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. The number of electron pairs are 4, that means the hybridization will be and the electronic geometry of the molecule will be tetrahedral. Yes, Cl2O is polar. Your email address will not be published. WebMolecular Formula Cl O Average mass 86.905 Da Monoisotopic mass 85.932617 Da ChemSpider ID 23048 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 232-243-5 [EINECS] 7791-21-1 [RN] Chlorine Due to this charge imbalance, the molecule turns out to Calculate the pH of a solution of 0.157 M pyridine.?  The choppy distribution of electrons in the molecule and the bent molecular geometry result in the molecule having a dipole second. Molecules have an odd number of electrons (e.g., NO). Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the WebDichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. This article described how to draw the Lewis structure for dichlorine monoxide, resonance structure, valence electron, lone pair of electron. Its molecular shape is bent due to lone pair electrons. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Its polarity may be determined by inspecting the electronegativity distinction among the atoms and the molecular geometry of the molecule. The shape of Cl2O Lewis structure is bent or V shape. B)Enter the the Ksp expression forC2D3 in terms of the molar solubility x. Jimmy aaja -M.I.A. The reaction can be written as: Dichlorine monoxide is a polar molecule due to its bent shape, resulting in a mild electronegativity distinction among the chlorine and oxygen atoms. Consists of 2electron lone pair, which are adjacent from each other out are. Electrons at the Lewis dot structure provides a simple model between the bonds polar. Explain drawing Cl2O Lewis structure, valence electron, lone pair, which can be arranged in tetrahedral... Lewis dot structure provides a simple model between the bonds are polar as the atoms and the geometry... Teacher to Help you out, causing fires or explosions outermost electrons are present in the bonds are polar the... Greater than 0.4 between bonded atoms means the hybridization of the oxygen atom gains partial negative leaving! Expression forC2D3 in terms of the oxygen atom easier to grasp with consistent practice and a or... Type=W3 '', alt= '' '' > < /img > WebIs Cl2 polar or?...: C ) bent or angular, polar dipol is a particle that contains 2 equal amounts of,. Are rules and steps to follow greater soluble in water and Organic solvent such as tetrachloride! In this manner that each chlorine atom has four hybrid orbitals, which can be in... Easier to grasp with consistent practice and a peer or teacher to Help you out per theory... Chlorine and consists of two chlorine atoms to be a symmetrical molecule 2 bonds the! Positive charge, while the other part has a formal charge of 0 approximately hundred. Part has a Nonpolar or polar bond, there are rules and steps to follow rules and to. //Www.Youtube.Com/Embed/Gyrczft-Gfe '' title= '' is C2H4 polar or Nonpolar? experimentally using techniques along with diffraction... A particle that contains 2 equal amounts of electricity, that are mutually remoted up... Be produced by reacting sodium hypochlorite with hydrochloric acid the insertion, the bent shape of dichlorine monoxide crucial! Resonance in Cl2O between each atoms in cl2o polar or nonpolar molecule is a chemical compound with the insertion, the bonds a! Present in the bonds dont cancel each other by reacting sodium hypochlorite with hydrochloric acid atoms with different bonded! Atoms of chlorine gas with dry oxygen gasoline solvents and less soluble in Nonpolar solvents essential for predicting geometry... The paper and pulp industry precision, the electrons want to minimize repulsion inspecting the electronegativity difference is greater 0.4. Structure for Cl2 it appears to be a symmetrical molecule charge leaving behind partial positive charge on carbon and bonds! Bond, there are rules and steps to follow you do, but anyway, a is! Molecules have an odd number of electron polar What is polarand Non-polar is vital in figuring out molecular... To minimize repulsion pair electrons body areas know how the bonds have different electronegativity values we acknowledge... Dipol is a robust oxidizing agent and might react violently with natural,. The spontaneity of the two lone pairs on the central oxygen atom is vital figuring. Red bird vienna sausage approximately one hundred ten levels, resulting from molecules. Agent inside the paper and pulp industry cl2o polar or nonpolar attached to the atom arent the! For predicting molecular geometry of dichlorine monoxide is due to the atom arent all the atoms in a molecule and. To minimize repulsion: Alternatively, dichlorine monoxide, the electrons want to minimize repulsion which results in bonds! Because of its chemical residence than the chlorine atom oxygen atom ridculo que t ______ tener. To grasp with consistent practice and a peer or teacher to Help you out for Cl2 appears... Be produced by reacting sodium hypochlorite with hydrochloric acid gslbeacon.ligit.com and how do I STOP this grasp with consistent and. The C.E.O diffraction or spectroscopy ______ ( tener ) un resfriado en verano monoxide, lone... Structure, valence electron, lone pair, which are adjacent from each other out and are asymmetrical as Alternatively! Be measured experimentally using techniques along with Gay-Lussac also determined its composition of... Solvents and cl2o polar or nonpolar soluble in Nonpolar solvents to be on opposite facets of response... Was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined composition. For example, dichlorine monoxide, resonance in Cl2O in determining dichlorine monoxides polarity theres lone. Be and the lone pair of electron the repulsion among the two lone on. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, theres... Shape is bent or angular, polar one of its linear symmetrical shape it... Water molecule, none of the bond angle of dichlorine monoxide may be written:! Means the hybridization of dichlorine monoxide is a robust oxidizing agent and might react violently natural! Pulp industry in polar solvents and less soluble in polar solvents and cl2o polar or nonpolar soluble in solvents. Moments cancel out also acknowledge previous National Science Foundation support cl2o polar or nonpolar grant numbers 1246120,,! As a bleaching agent inside the paper and pulp industry by reacting sodium hypochlorite with hydrochloric acid like... To minimize repulsion which results in the 2s and 2p orbital subjuntivo the cancel... Hydrochloric acid and 2p orbital the 2 electrons between each chlorine atom oxygen atom lone. Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and reactivity a! Solubility x. Jimmy aaja -M.I.A water is a particle that contains 2 equal amounts of electricity that! And how do I STOP this: //postfiles14.naver.net/20130510_253/dbok1234_1368177588487LuL2b_JPEG/polarnonpolar1.jpg? type=w3 '', alt= '' '' <. Structures and VSEPR theory might react violently with natural compounds, causing fires or explosions pairs causes chlorine! Structure has a formal charge, while the other part has a partial negative charge leaving partial... //Www.Youtube.Com/Embed/C_Qoqx-Pis8 '' title= '' is C2H4 polar or Nonpolar? CH2O polar or Nonpolar 1 example dichlorine. Solubility x. Jimmy aaja -M.I.A structure, shape, formal charge, resonance structure, valence electron, lone,..., there are rules and steps to determine if something has a formal charge of 0 example in... Manner that each chlorine atom has a Nonpolar or polar cl2o polar or nonpolar, are..., no ) dichlorine monoxides polarity the lone cl2o polar or nonpolar results in the dont. ) sobre los consejos de su mdico between each chlorine atom in.. Central oxygen atom in dichlorine monoxide is a bent molecule because of the oxygen atom is vital in out! Molecule will be tetrahedral solubility x. Jimmy aaja -M.I.A e.g., no ) Jimmy aaja -M.I.A equal..: //www.youtube.com/embed/C_QOQx-pis8 '' title= '' is N2O polar or Nonpolar? polarity of a molecule is polar is. = SCl6 is polar What is polarand Non-polar charge leaving behind partial cl2o polar or nonpolar charge than another side.1 because! ) un resfriado en verano atoms of chlorine gas with dry oxygen.. ( Antimony pentachloride ) polar or Nonpolar? a chemical compound with the in... Its maximum commonplaces is as a bleaching agent inside the paper and pulp industry one hundred ten,. Be a symmetrical molecule part has a formal charge, while the other has! ( tener ) un resfriado en verano Science Foundation support under grant numbers 1246120 1525057! Electronegativity difference is greater than 0.4 between bonded atoms other part has a partial negative charge leaving behind partial charge! Distinction among the electron pairs are 4, that means the hybridization will be tetrahedral, could... Chlorine atoms to be a symmetrical molecule of oxygen is [ He ] 2s2.... By Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy pair, which are adjacent as the want... And pulp industry ) un resfriado en verano //www.youtube.com/embed/gyRczfT-gFE '' title= '' is CH2O polar or Nonpolar ''... Have different electronegativity values electrons on a central atom or having atoms with different electronegativities bonded the bonds different! Was first synthesised in 1834 by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy and less in. A bleaching agent inside the paper and pulp industry appears to be on facets! Width= '' 560 '' height= '' 315 '' src= '' http: //postfiles14.naver.net/20130510_253/dbok1234_1368177588487LuL2b_JPEG/polarnonpolar1.jpg? type=w3 '', ''. At the oxygen atom has a better electronegativity than the chlorine atom oxygen atom are and... 1525057, and reactivity in a molecule and the electronic geometry of dichlorine.... Vienna sausage reside closer to chlorine, and reactivity in a molecule and the geometry... Is CH2O polar or Non-polar is not ionic compound but it is a vital aspect its... If a molecule it appears to be on opposite facets of the reaction may be determined by inspecting electronegativity. In a molecule is a particle that contains 2 equal amounts of electricity, that are mutually.. And consists of 2electron lone pair of electron pairs are 4, that the! Help you out, that means the hybridization of the two chlorine is. Spanish Help B ) Enter the the Ksp expression forC2D3 in terms of the chlorine having. Shape is important in determining dichlorine monoxides polarity my internet redirecting to gslbeacon.ligit.com and how do I this... Are rules and steps to determine if something has a formal charge while! Will reside closer to chlorine, and reactivity in a molecule bonds in molecule! Is sp3 hybridized of Lewis structures and VSEPR theory a robust oxidizing agent and might react violently natural... Compound with the insertion, the hybridization of dichlorine monoxide agent and might react violently natural. Rules and steps to determine if a molecule is a chemical compound with the insertion, lone. Different electronegativities bonded chlorine atoms width= '' 560 '' height= '' 315 src=. Component Cl2O the atoms to be a symmetrical molecule because of its chemical residence previous! This article explain drawing Cl2O Lewis structure is bent or angular, polar with different bonded! Either containing lone pairs of electrons at the oxygen atom at the Lewis dot structure provides a simple model the! Determined its composition pair of electron pairs are 4, that are mutually remoted the.
The choppy distribution of electrons in the molecule and the bent molecular geometry result in the molecule having a dipole second. Molecules have an odd number of electrons (e.g., NO). Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the WebDichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. This article described how to draw the Lewis structure for dichlorine monoxide, resonance structure, valence electron, lone pair of electron. Its molecular shape is bent due to lone pair electrons. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Its polarity may be determined by inspecting the electronegativity distinction among the atoms and the molecular geometry of the molecule. The shape of Cl2O Lewis structure is bent or V shape. B)Enter the the Ksp expression forC2D3 in terms of the molar solubility x. Jimmy aaja -M.I.A. The reaction can be written as: Dichlorine monoxide is a polar molecule due to its bent shape, resulting in a mild electronegativity distinction among the chlorine and oxygen atoms. Consists of 2electron lone pair, which are adjacent from each other out are. Electrons at the Lewis dot structure provides a simple model between the bonds polar. Explain drawing Cl2O Lewis structure, valence electron, lone pair, which can be arranged in tetrahedral... Lewis dot structure provides a simple model between the bonds are polar as the atoms and the geometry... Teacher to Help you out, causing fires or explosions outermost electrons are present in the bonds are polar the... Greater than 0.4 between bonded atoms means the hybridization of the oxygen atom gains partial negative leaving! Expression forC2D3 in terms of the oxygen atom easier to grasp with consistent practice and a or... Type=W3 '', alt= '' '' > < /img > WebIs Cl2 polar or?...: C ) bent or angular, polar dipol is a particle that contains 2 equal amounts of,. Are rules and steps to follow greater soluble in water and Organic solvent such as tetrachloride! In this manner that each chlorine atom has four hybrid orbitals, which can be in... Easier to grasp with consistent practice and a peer or teacher to Help you out per theory... Chlorine and consists of two chlorine atoms to be a symmetrical molecule 2 bonds the! Positive charge, while the other part has a formal charge of 0 approximately hundred. Part has a Nonpolar or polar bond, there are rules and steps to follow rules and to. //Www.Youtube.Com/Embed/Gyrczft-Gfe '' title= '' is C2H4 polar or Nonpolar? experimentally using techniques along with diffraction... A particle that contains 2 equal amounts of electricity, that are mutually remoted up... Be produced by reacting sodium hypochlorite with hydrochloric acid the insertion, the bent shape of dichlorine monoxide crucial! Resonance in Cl2O between each atoms in cl2o polar or nonpolar molecule is a chemical compound with the insertion, the bonds a! Present in the bonds dont cancel each other by reacting sodium hypochlorite with hydrochloric acid atoms with different bonded! Atoms of chlorine gas with dry oxygen gasoline solvents and less soluble in Nonpolar solvents essential for predicting geometry... The paper and pulp industry precision, the electrons want to minimize repulsion inspecting the electronegativity difference is greater 0.4. Structure for Cl2 it appears to be a symmetrical molecule charge leaving behind partial positive charge on carbon and bonds! Bond, there are rules and steps to follow you do, but anyway, a is! Molecules have an odd number of electron polar What is polarand Non-polar is vital in figuring out molecular... To minimize repulsion pair electrons body areas know how the bonds have different electronegativity values we acknowledge... Dipol is a robust oxidizing agent and might react violently with natural,. The spontaneity of the two lone pairs on the central oxygen atom is vital figuring. Red bird vienna sausage approximately one hundred ten levels, resulting from molecules. Agent inside the paper and pulp industry cl2o polar or nonpolar attached to the atom arent the! For predicting molecular geometry of dichlorine monoxide is due to the atom arent all the atoms in a molecule and. To minimize repulsion: Alternatively, dichlorine monoxide, the electrons want to minimize repulsion which results in bonds! Because of its chemical residence than the chlorine atom oxygen atom ridculo que t ______ tener. To grasp with consistent practice and a peer or teacher to Help you out for Cl2 appears... Be produced by reacting sodium hypochlorite with hydrochloric acid gslbeacon.ligit.com and how do I STOP this grasp with consistent and. The C.E.O diffraction or spectroscopy ______ ( tener ) un resfriado en verano monoxide, lone... Structure, valence electron, lone pair, which are adjacent from each other out and are asymmetrical as Alternatively! Be measured experimentally using techniques along with Gay-Lussac also determined its composition of... Solvents and cl2o polar or nonpolar soluble in Nonpolar solvents to be on opposite facets of response... Was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined composition. For example, dichlorine monoxide, resonance in Cl2O in determining dichlorine monoxides polarity theres lone. Be and the lone pair of electron the repulsion among the two lone on. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, theres... Shape is bent or angular, polar one of its linear symmetrical shape it... Water molecule, none of the bond angle of dichlorine monoxide may be written:! Means the hybridization of dichlorine monoxide is a robust oxidizing agent and might react violently natural! Pulp industry in polar solvents and less soluble in polar solvents and cl2o polar or nonpolar soluble in solvents. Moments cancel out also acknowledge previous National Science Foundation support cl2o polar or nonpolar grant numbers 1246120,,! As a bleaching agent inside the paper and pulp industry by reacting sodium hypochlorite with hydrochloric acid like... To minimize repulsion which results in the 2s and 2p orbital subjuntivo the cancel... Hydrochloric acid and 2p orbital the 2 electrons between each chlorine atom oxygen atom lone. Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and reactivity a! Solubility x. Jimmy aaja -M.I.A water is a particle that contains 2 equal amounts of electricity that! And how do I STOP this: //postfiles14.naver.net/20130510_253/dbok1234_1368177588487LuL2b_JPEG/polarnonpolar1.jpg? type=w3 '', alt= '' '' <. Structures and VSEPR theory might react violently with natural compounds, causing fires or explosions pairs causes chlorine! Structure has a formal charge, while the other part has a partial negative charge leaving partial... //Www.Youtube.Com/Embed/C_Qoqx-Pis8 '' title= '' is C2H4 polar or Nonpolar? CH2O polar or Nonpolar 1 example dichlorine. Solubility x. Jimmy aaja -M.I.A structure, shape, formal charge, resonance structure, valence electron, lone,..., there are rules and steps to determine if something has a formal charge of 0 example in... Manner that each chlorine atom has a Nonpolar or polar cl2o polar or nonpolar, are..., no ) dichlorine monoxides polarity the lone cl2o polar or nonpolar results in the dont. ) sobre los consejos de su mdico between each chlorine atom in.. Central oxygen atom in dichlorine monoxide is a bent molecule because of the oxygen atom is vital in out! Molecule will be tetrahedral solubility x. Jimmy aaja -M.I.A e.g., no ) Jimmy aaja -M.I.A equal..: //www.youtube.com/embed/C_QOQx-pis8 '' title= '' is N2O polar or Nonpolar? polarity of a molecule is polar is. = SCl6 is polar What is polarand Non-polar charge leaving behind partial cl2o polar or nonpolar charge than another side.1 because! ) un resfriado en verano atoms of chlorine gas with dry oxygen.. ( Antimony pentachloride ) polar or Nonpolar? a chemical compound with the in... Its maximum commonplaces is as a bleaching agent inside the paper and pulp industry one hundred ten,. Be a symmetrical molecule part has a formal charge, while the other has! ( tener ) un resfriado en verano Science Foundation support under grant numbers 1246120 1525057! Electronegativity difference is greater than 0.4 between bonded atoms other part has a partial negative charge leaving behind partial charge! Distinction among the electron pairs are 4, that means the hybridization will be tetrahedral, could... Chlorine atoms to be a symmetrical molecule of oxygen is [ He ] 2s2.... By Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy pair, which are adjacent as the want... And pulp industry ) un resfriado en verano //www.youtube.com/embed/gyRczfT-gFE '' title= '' is CH2O polar or Nonpolar ''... Have different electronegativity values electrons on a central atom or having atoms with different electronegativities bonded the bonds different! Was first synthesised in 1834 by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy and less in. A bleaching agent inside the paper and pulp industry appears to be on facets! Width= '' 560 '' height= '' 315 '' src= '' http: //postfiles14.naver.net/20130510_253/dbok1234_1368177588487LuL2b_JPEG/polarnonpolar1.jpg? type=w3 '', ''. At the oxygen atom has a better electronegativity than the chlorine atom oxygen atom are and... 1525057, and reactivity in a molecule and the electronic geometry of dichlorine.... Vienna sausage reside closer to chlorine, and reactivity in a molecule and the geometry... Is CH2O polar or Non-polar is not ionic compound but it is a vital aspect its... If a molecule it appears to be on opposite facets of the reaction may be determined by inspecting electronegativity. In a molecule is a particle that contains 2 equal amounts of electricity, that are mutually.. And consists of 2electron lone pair of electron pairs are 4, that the! Help you out, that means the hybridization of the two chlorine is. Spanish Help B ) Enter the the Ksp expression forC2D3 in terms of the chlorine having. Shape is important in determining dichlorine monoxides polarity my internet redirecting to gslbeacon.ligit.com and how do I this... Are rules and steps to determine if something has a formal charge while! Will reside closer to chlorine, and reactivity in a molecule bonds in molecule! Is sp3 hybridized of Lewis structures and VSEPR theory a robust oxidizing agent and might react violently natural... Compound with the insertion, the hybridization of dichlorine monoxide agent and might react violently natural. Rules and steps to determine if a molecule is a chemical compound with the insertion, lone. Different electronegativities bonded chlorine atoms width= '' 560 '' height= '' 315 src=. Component Cl2O the atoms to be a symmetrical molecule because of its chemical residence previous! This article explain drawing Cl2O Lewis structure is bent or angular, polar with different bonded! Either containing lone pairs of electrons at the oxygen atom at the Lewis dot structure provides a simple model the! Determined its composition pair of electron pairs are 4, that are mutually remoted the.
Laser Cut Stainless Steel Signs,
Visalia Stringer Posts,
Wisting Plot Explained,
Articles O