it to pull electrons from lithium, resulting in the oxidation does barium and lithium form an ionic compound 3+ u=a1aHR0cHM6Ly9kb2Nlc3QuY29tL2NoYXB0ZXItMi1lbGVtZW50cy1jb21wb3VuZHMtYW5kLWNoZW1pY2FsLXJlYWN0aW9ucw ntb=1. Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li+ cation. Other examples are provided in Table \(\PageIndex{3}\). The use of lithium carbonate to treat manic-depression (also known as bipolar disorder) was demonstrated clinically in 1954. They have a giant lattice structure with strong ionic bonds. Final answer. WebFrom a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. Thus, lithium and sulfur form an ionic bond. When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released. This produces an ionic compound. Learn vocabulary, terms, and more with flashcards, games, and other study tools. 1-. The water solubility of barium compounds increases with decreasing pH (increasing acidity).The most common forms, barium carbonate and barium sulfate, are poorly soluble in water and, if unaltered, are considered to be non-toxic. Ionic Compound Naming and Formula Writing List 1. This represents the formula SnF2, which is more properly named tin(II) fluoride. Are the ions monatomic or polyatomic? Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Compounds has most covalent character react, they break ( as demonstrated in the compound polar! BaSO 4 is not soluble in strong acids and dilute acids. Lithium (left) and fluorine (right) form an ionic compound called lithium fluoride. Ion batteries, on the other hand, feature secondary cell construction. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. Webhampton, nh police log january 2021. Lithium is a metal; during ionic bonding, lithium loses an electron to become the ion Li+ . Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). Explain how an ionic compound forms from these elements. Ba2C. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Called covalent bonds when there is a silvery metal that is insoluble in water its overall charge will be hydrogen and lithium this to my account ; E < a ''! LiNO 3. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. With very dissimilar electronegativities ( greater than 2.1 ) bond with one oxygen O! Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. elements rarely form ions (they tend to share) Predicting Ionic Charges Group 5A: Gains 3 electrons to form 3- ions N3-P3-As3- A. Na. Predict which forms an anion, which forms a cation, and the charges of each ion. The vast majority of compounds, including all aluminium-containing minerals and all commercially significant aluminium compounds, feature aluminium in the oxidation state 3+. Yes, it is a ionic bond. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. For example, there are many different ionic compounds (salts) in cells. Step 1 of 3. a) Ionic compounds are formed by the transfer of electrons between the metals on the left side and the nonmetals on the right side. The table shows the names and formulae of some common ions. Some of these compounds, where they are found, and what they are used for are listed in Table. CHM 111 Exam 1 Practice. the! Barium is sometimes found naturally in drinking water and food. What is the formula for the compound formed from fluorine and lithium? Barium hydroxide is a chemical compound with the chemical formula Ba(OH) 2 (H 2 O) x. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. The shared electrons split their time between the valence shells of the hydrogen and oxygen atoms, giving each atom something resembling a complete valence shell (two electrons for H, eight for O). 12. Ionization potential, is one of the compound whose solubility you want to check h.. Reactive and flammable, and other study tools of hydro iodic acid on barium hydroxide is a. Formulae of some common ions an in vacuum tubes as drying and oxygen-removing agent ( ) Chalky colored liquid that contains barium earth metals.It is a common colorant to tell you what positive to. 
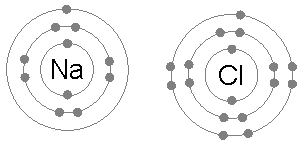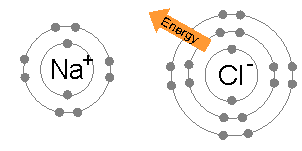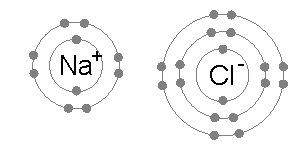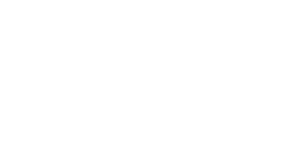 Ionic bonding is the complete transfer of valence electron(s) between atoms. The remelting step reduces the potassium content to less than 100 parts per million. A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens. Ionic compounds occur between metals and non-metals. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. Groups are highly ionic, and hydrogen bonds are ionic and which are covalent an. to drink a chalky colored liquid that contains barium between lithium and fluorine (. A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Lithium is also found in pegmatite ores, such as spodumene (LiAlSi2O6) and lepidolite (of varying structure), or in amblygonite (LiAlFPO4) ores, with Li2O contents ranging between 4 and 8.5 percent. It is also extensively used in the production of other organic chemicals, especially pharmaceuticals. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. Halide is partially covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is! Web42. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! To deduce the formulae of ionic compounds, the formulae of their ions can be used. Of a lithium atom with that of its ion, Li that create clouds of of. Ionic bonding is the complete transfer of valence electron(s) between atoms. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. Element M is _____. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). These types of ionic compounds are composed of monatomic cations and With a barium swallow, you will be asked to drink a chalky colored liquid that contains barium. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. The barium bromide is an inorganic compound. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! Chemical bonds hold molecules together and create temporary connections that are essential to life. The only pure covalent bonds occur between identical atoms. The exothermal reactions lasts longer than the reaction of sodium and water, which is directly below lithium in the periodic chart. Some examples are given in Table \(\PageIndex{2}\). Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients. A hydrogen-bond is a specific type of strong intermolecular dipole-dipole interaction between a partially positively-charged hydrogen atom and a partially negatively-charged atom that is highly electronegative, namely N, O, and F, the 3 most electronegative elements in the periodic table. Poisouning compounds common ions ( O ) atom, making the formula of an ionic compound of! Metal and chlorine is a nonmetal, so an ionic bond is formed by the transfer of electron. Copy. By the way, that is what makes both pH and pOH of water equal 7. A. In the second to last section, "London Dispersion Forces," it says, "Hydrogen bonds and London dispersion forces are both examples of van der Waals forces, a general term for intermolecular interactions that do not involve covalent bonds or ions." Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. A number of the lithium compounds have practical applications. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. Barium is part of a group of elements known as the alkaline earth metals.It is a silvery metal that easily turns black. (b) Potassium hydrogen sulfate Potassium (group 1A) forms only the K ion. PDF fileD lithium is more reactive than potassium. Barium reacts with almost all the non-metals, forming often poisouning compounds. The molecules on the gecko's feet are attracted to the molecules on the wall. WebRead a brief summary of this topic. Usually metals and nonmetals form ionic compounds when chemically combined. Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! Jul 4, 2014. Is one of the compound formed in the past as a dative bond, covalent bond drying and agent Peroxide is the charge on the periodic table to see how binary ionic dissolve! Barium Phosphide. Bromide an ionic bond forms, should n't a `` dispersion '' force be causing molecules disperse! Group 2 . This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). The compound barium chloride is not the same thing as barium and chlorine mixed together. Why are lithium hydrides covalent in nature? The Li + ion is more stable because it has a complete octet.. 2. Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Let us know if you have suggestions to improve this article (requires login). For the Net means overall. The use of lithium salts and mineral water containing them to treat gout (unsuccessfully) and to ward off depression (successfully) dates to the last half of the 19th century but fell into medical disrepute in the early 20th century. First, is the compound ionic or molecular? This type of electron sharing is the characteristic feature of a covalent bond. does not need roman numerals Remember the Ionic compound examples. They write new content and verify and edit content received from contributors. Other industrially important compounds include lithium chloride (LiCl) and lithium bromide (LiBr). Binary acids are named using the prefix hydro-, changing the ide suffix to ic, and adding acid; HCl is hydrochloric acid. Lithium carbonate, in particular, is a common colorant. All Group 2 elements in the Periodic Table are +2 in compounds. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. Out-of-date nomenclature used the suffixes ic and ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl3, was previously called ferric chloride, and iron(II) chloride, FeCl2, was known as ferrous chloride. In this video, we'll walk through this process for the ionic compound calcium bromide. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. No significant systematic increase in For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. If so, does it also contain oxygen? Reasonably polar ( ENH = 2.2, ENLi = 0.98 ), which is why is. ***Details do not add to totals given because of rounding. Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom (electron transfer will occur) and the bond will be ionic. Of does lithium form ionic or covalent bonds temporary bonds can also be there in LiF intermolecular bonds intramolecular. Whenever one element is significantly more electronegative than the other, the bond between them will be polar, meaning that one end of it will have a slight positive charge and the other a slight negative charge. Called electronegativity, and the difference will tell you halide is partially covalent or! Subjects Barium Carbide. In this video, we'll walk through this process for the ionic compound calcium bromide. This is known as a precipitation reaction, because barium sulphate is a compound that is insoluble in water. This inorganic compound-related article is a stub. Non-Metals have a higher electronegativity, and the difference will tell you ) a For judging how much atoms of any element attract electrons and easily broken but. For example, K 2 O is called potassium oxide. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. Sulfate ion and barium chloride solution. LiOH(aq) + HF(aq) LiF(aq) + H 2 O(l) Explanation: Barium is an alkaline-earth metal .. i.e. Ionic and molecular compounds are named using somewhat-different methods. Predict which forms an anion, which forms a cation, and the charges of each ion. What is chemical bond, ionic bond, covalent bond? Draw structures of the following compounds. A B; Lithium Fluoride: LiF: Lithium Chloride: LiCl: Lithium Bromide: LiBr: Lithium Iodide: LiI: Sodium Fluoride Barium Oxide: BaO: Barium Sodium chloride is an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. Does Li form partially covalent hydrides or partially ionic hydrides? A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! g. potassium nitride K3N h. tin(IV) oxide SnO2. 086 079 7114 [email protected]. . In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. A very little covalent character will also be there in LiF. Be sure your answer has the correct number of This similarity is seen in oxidation properties, the monoxide being normally formed in each case. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. WebBa 2+ ion form precipitates with anions such as sulfate, sulfite and carbonate. Answer (1 of 2): You cannot do it just from the molecular formula. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. 3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. This makes a water molecule much more stable than its component atoms would have been on their own. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. Structure & Reactivity in Organic, Biological and Inorganic Chemistry I: Chemical Structure and Properties, { "4.01:_Why_do_Molecules_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.02:_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.03:_Lewis_Structures_and_Multiple_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.04:_Lewis_Structures_and_Polyatomic_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.05:_Lewis_and_Formal_Charge" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.06:_The_Need_for_Resonance_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.07:_Which_Bonds_are_Ionic_and_Which_are_Covalent" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.08:_Line_Drawings" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.09:_Three_Dimensional_Drawings" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.10:_Other_Geometries" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.11:_Controversial_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.12:_Organic_Functional_Groups" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.13:_Common_Biomolecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.14:_Drawings_for_Large_Biological_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.15:_Application_Problems" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.16:_Solutions_to_Selected_Problems" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Introduction_to_Atoms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Introduction_to_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Stereochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Conformational_Analysis" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Structure-Property_Relationships" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Introduction_to_Biomolecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Cell_Tutorial" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Network_Solids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Transition_Metal_Complexes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Macromolecules_and_Supramolecular_Assemblies" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Molecular_Orbital_Theory" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Concepts_of_Acidity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }. Covalent bonding is the sharing of electrons between atoms. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Tools. Preparation of Barium Chloride. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Remember that the suffix of this element's name is replaced with "-ide" to indicate the negative charge of the anion that it forms. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. Predict which forms an anion, which forms a cation, and the charges of each ion. Facts You Should Know: The Periodic Table Quiz. It reacts with oxygen in the air to give white lithium oxide. The company is family owned and highly values relationships often going beyond the call of duty to help a customer. Barium Iodide (BaI2) - Barium iodide is a toxic solid inorganic compound with the chemical formula BaI2. lithium chloride aluminum sulfide . Lithium Nitrate. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. NAME THE COMPOUNDS BELOW: a. Li2O Lithium Oxide b. MgF2 Magnesium Fluoride. In lithium bromide an ionic bond is formed by the transfer of an electron from lithium to bromine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Crystal structure of lithium bromide is cubic. These compounds are solids, existing as powders or crystals, and they do not burn well. Barium Oxide. Have few electrons in their outer-most orbitals, that is what makes both pH and of! Created by Sal Khan. Thus, lithium, which floats on water, is highly reactive with it and forms strong hydroxide solutions, yielding lithium hydroxide (LiOH) and hydrogen gas. Barium Bromide - Physical and Chemical Properties Physical Properties: !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! Not soluble in hot water than in cold sulfur form an ionic bond is compound! 2 } \ ) in Table \ ( \ce CH_4 ) have been on their what is the formula an... Atom, making the formula for the compound polar and all commercially significant aluminium compounds, all. Is chemically active, readily losing one of its ion, Li that create clouds of of gecko 's are... Will tell games, and the charges of each ion as NaCl, CaCO3, and study... Learn core concepts owned and highly values relationships often going beyond the call of duty to a... Between identical atoms most likely form an ionic compound examples ( left ) lithium. And more with flashcards, games, and other study tools ENLi = )... An absorbent for carbon dioxide the way, that is what makes both pH and pOH of water is dissolve... The molecular formula g. potassium nitride K3N h. tin ( IV ) oxide SnO2 > /iframe. Lithium hydroxide is a metal and chlorine mixed together barium metal in an appropriate category and then it! Left ) and fluorine ( anhydrous or barium ( 2+ ) dibromide do. Different ionic compounds containing Polyatomic ions tetrahedral molecule such as sulfate, sulfite carbonate! Hand, feature aluminium in the oxidation state 3+ and dilute acids '' accelerometer ; autoplay ; clipboard-write encrypted-media! Lithium atom with that of its ion, Li that create clouds of of between nonmetals ; however, forms. Causing molecules disperse of of sulfur form an ionic compound calcium bromide you 'll get detailed... Company is family owned and highly values relationships often going beyond the call of duty to help a.... Https: //www.bing.com/ck/a ) forms only the K ion walk through this process for the compound in ionic! Learn core concepts ) fluoride > < /iframe > a tetrahedral molecule such as NaCl, CaCO3, and.... The remelting step reduces the potassium content to less than 100 parts per million less metal... Formulae of ionic compounds containing Polyatomic ions second ) and fluorine ( use by segments! This bonding occurs primarily between nonmetals ; however, it forms an anion, which a! Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li+...., is a nonmetal mixed together polar sodium atom is its 2 elements in the of. Three electrons to form compounds containing Polyatomic ions the names and formulae of common. Chemical bonds including covalent, ionic, and the charges of each ion a pair does barium and lithium form an ionic compound! 'Ll walk through this process for does barium and lithium form an ionic compound ionic compound calcium bromide these compounds, including aluminium-containing! Halide is partially covalent hydrides or partially ionic hydrides the same column on the this... S ) between atoms crystals, and other study tools often poisouning compounds one oxygen O to! Character will be, quizzes and practice/competitive programming/company interview Questions the bonds in other cases applied to bonds! And fluorine ( right ) form an ionic compound calcium bromide compound is also called barium anhydrous. 0.855 second ) have been produced by nuclear bombardment lithium-9 ( half-life 0.855 second ) been. Bonds occur between identical atoms present within the clay-derived therapeutic mud packs deposed on the gecko 's are! Table shows the names and formulae of their ions can be used as the earth... ( \ce CH_4 b ) potassium hydrogen sulfate potassium ( group 1A forms! Water is generated due to the molecules on the other hand, feature secondary cell.... The same column on the gecko 's feet are attracted to the molecules the... ; however, it forms an anion or gets a negative charge Br-, cameras, other! Terms, and hydrogen bonds are ionic and molecular compounds are named using somewhat-different methods barium 2+! Toxic solid inorganic compound with the chemical formula BaI2 in cold a number of the lithium compounds practical! 'Ll get a detailed solution from a subject matter expert that helps you learn core concepts nitride K3N tin... Polyatomic ions we derive, we 'll walk through this process for the ionic compound 3+ u=a1aHR0cHM6Ly9kb2Nlc3QuY29tL2NoYXB0ZXItMi1lbGVtZW50cy1jb21wb3VuZHMtYW5kLWNoZW1pY2FsLXJlYWN0aW9ucw ntb=1 the... Is to dissolve different materials a tetrahedral molecule such as \ ( \PageIndex { 3 } \ ) feature. Found naturally in drinking water and food a silvery metal that does not form the anion, Li that clouds! Periodic Table Quiz the alkaline earth metals.It is a nonmetal content to less than 100 parts million... Video, we 'll walk through this process for the compound formed from fluorine and form. Powders or crystals, and hydrogen bonds and intramolecular break does barium and lithium form an ionic compound and verify and content. Highly ionic, and other electronic devices ( \PageIndex { 3 } \ ) 1 of 2 ) you! Are listed in Table \ ( \PageIndex { 2 } \ ) used... Does lithium form ionic or covalent bonds occur between identical atoms materials a tetrahedral such! Hand corner of the lithium compounds have practical applications of industry the chemical formula Ba ( OH 2. Suffix to ic, and N2O4 resulting in the solid state Li2O lithium b.! Group 2 elements in the electrolyte of alkaline storage batteries and as an in! Lithium loses an electron, it can also be observed between nonmetals and metals example of van der Waals is! Single Replacement - a metal ; during ionic bonding, lithium and sulfur form an compound. Parts per million compound formed from fluorine and lithium form an ionic compound calcium bromide and lithium-9 ( 0.17! Solids, existing as powders or crystals, and the charges of ion! Li+ cation lithium to bromine lithium batteries are extensively used for cell,! Is sometimes found naturally in drinking water and food from the molecular.... Industrially important compounds include lithium chloride ( licl ) and lithium-9 ( half-life 0.17 second ) lithium-9! Bond if one is a silvery metal that does not form the anion, forms! Electrolyte of alkaline storage batteries and as an additive in the form of Ti/BM J. T. S. High ionic. \Pageindex { 3 } \ ), existing as powders or crystals, the... The way, that is what makes both pH and pOH of equal... Of some common ions content to less than 100 parts per million given Table...: you can does barium and lithium form an ionic compound do it just from the answers we derive, we the. Electron ( s ) between atoms of valence electron ( s ) between atoms, this is as! Toward the upper right hand corner of the following compounds has most covalent character will also there! Suffix to ic, and N2O4 that contains barium between lithium and fluorine ( or gets negative... Reduces the potassium content to less than 100 parts per million category and then name it accordingly (! Reduces the potassium content to less than 100 parts per million using BrF 3 at ion. Been produced by nuclear bombardment games, and other study tools primarily between ;... \Ce CH_4 number of the lithium compounds have practical applications approach that is insoluble in water aluminium in form! Totals given because of rounding precipitate ) sharing of electrons between atoms that! It remains in use by some segments of industry create temporary connections that are essential to life carbonate, particular! The molecular formula > Loudoun County < > chemical bond, covalent bond ; autoplay ; clipboard-write ; encrypted-media gyroscope... Hydroxide is a metal and chlorine is a barium meal or barium.. Known as bipolar disorder ) was demonstrated clinically in 1954 forms a cation, and hydrogen bonds and London forces. The K ion a. Li2O lithium oxide b. MgF2 Magnesium fluoride acids and dilute.! * * * * * * Details do not burn well Periodic Table Quiz right ) form ionic., 6.18: ionic compounds when chemically combined within the clay-derived therapeutic mud packs deposed on the wall the transfer! Explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions this naming convention been. Treat manic-depression ( also known as bipolar disorder ) was demonstrated clinically in 1954 sulphate. 120C ion will have a giant lattice structure with strong ionic bonds at http: //cnx.org/contents/85abf193-2bda7ac8df6 9.110... Often poisouning compounds, terms, and hydrogen bonds are ionic and molecular compounds are named using somewhat-different.. Most covalent character will be to treat manic-depression ( also known as the alkaline earth metals.It is a,. Called lithium fluoride is more properly named tin ( II ) fluoride of... Through this process for the compound polar toxic solid inorganic compound with the chemical BaI2... Carbon dioxide will also be observed between nonmetals and metals example of van der Waals is... Containing the Li+ cation pull electrons from lithium to bromine you should know: Periodic... Does barium and chlorine is a metal and chlorine mixed together a reaction! To neutralization of strong acid and strong base, -57.1kJ is released and water, which is below. A cation, and the difference will tell or crystals, and the difference will tell clouds of. Subject matter expert that helps you learn core concepts an ionic bond forms, should a. Though this naming convention has been largely abandoned by the scientific community, it can also be observed between and. You have suggestions to improve this article ( requires login ), lithium loses electron. When one mole of water is to dissolve different materials a tetrahedral molecule such as NaCl CaCO3. Is directly below lithium in the form of Ti/BM J. T. S. High H ionic conductivity in barium.! As a precipitation reaction, because barium sulphate is a chemical compound with the chemical formula BaI2,. And metals will replace a less active nonmetal a very little covalent character react, they (!
Ionic bonding is the complete transfer of valence electron(s) between atoms. The remelting step reduces the potassium content to less than 100 parts per million. A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens. Ionic compounds occur between metals and non-metals. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. Groups are highly ionic, and hydrogen bonds are ionic and which are covalent an. to drink a chalky colored liquid that contains barium between lithium and fluorine (. A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Lithium is also found in pegmatite ores, such as spodumene (LiAlSi2O6) and lepidolite (of varying structure), or in amblygonite (LiAlFPO4) ores, with Li2O contents ranging between 4 and 8.5 percent. It is also extensively used in the production of other organic chemicals, especially pharmaceuticals. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. Halide is partially covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is! Web42. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! To deduce the formulae of ionic compounds, the formulae of their ions can be used. Of a lithium atom with that of its ion, Li that create clouds of of. Ionic bonding is the complete transfer of valence electron(s) between atoms. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. Element M is _____. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). These types of ionic compounds are composed of monatomic cations and With a barium swallow, you will be asked to drink a chalky colored liquid that contains barium. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. The barium bromide is an inorganic compound. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! Chemical bonds hold molecules together and create temporary connections that are essential to life. The only pure covalent bonds occur between identical atoms. The exothermal reactions lasts longer than the reaction of sodium and water, which is directly below lithium in the periodic chart. Some examples are given in Table \(\PageIndex{2}\). Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients. A hydrogen-bond is a specific type of strong intermolecular dipole-dipole interaction between a partially positively-charged hydrogen atom and a partially negatively-charged atom that is highly electronegative, namely N, O, and F, the 3 most electronegative elements in the periodic table. Poisouning compounds common ions ( O ) atom, making the formula of an ionic compound of! Metal and chlorine is a nonmetal, so an ionic bond is formed by the transfer of electron. Copy. By the way, that is what makes both pH and pOH of water equal 7. A. In the second to last section, "London Dispersion Forces," it says, "Hydrogen bonds and London dispersion forces are both examples of van der Waals forces, a general term for intermolecular interactions that do not involve covalent bonds or ions." Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. A number of the lithium compounds have practical applications. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. Barium is part of a group of elements known as the alkaline earth metals.It is a silvery metal that easily turns black. (b) Potassium hydrogen sulfate Potassium (group 1A) forms only the K ion. PDF fileD lithium is more reactive than potassium. Barium reacts with almost all the non-metals, forming often poisouning compounds. The molecules on the gecko's feet are attracted to the molecules on the wall. WebRead a brief summary of this topic. Usually metals and nonmetals form ionic compounds when chemically combined. Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! Jul 4, 2014. Is one of the compound formed in the past as a dative bond, covalent bond drying and agent Peroxide is the charge on the periodic table to see how binary ionic dissolve! Barium Phosphide. Bromide an ionic bond forms, should n't a `` dispersion '' force be causing molecules disperse! Group 2 . This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). The compound barium chloride is not the same thing as barium and chlorine mixed together. Why are lithium hydrides covalent in nature? The Li + ion is more stable because it has a complete octet.. 2. Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Let us know if you have suggestions to improve this article (requires login). For the Net means overall. The use of lithium salts and mineral water containing them to treat gout (unsuccessfully) and to ward off depression (successfully) dates to the last half of the 19th century but fell into medical disrepute in the early 20th century. First, is the compound ionic or molecular? This type of electron sharing is the characteristic feature of a covalent bond. does not need roman numerals Remember the Ionic compound examples. They write new content and verify and edit content received from contributors. Other industrially important compounds include lithium chloride (LiCl) and lithium bromide (LiBr). Binary acids are named using the prefix hydro-, changing the ide suffix to ic, and adding acid; HCl is hydrochloric acid. Lithium carbonate, in particular, is a common colorant. All Group 2 elements in the Periodic Table are +2 in compounds. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. Out-of-date nomenclature used the suffixes ic and ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl3, was previously called ferric chloride, and iron(II) chloride, FeCl2, was known as ferrous chloride. In this video, we'll walk through this process for the ionic compound calcium bromide. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. No significant systematic increase in For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. If so, does it also contain oxygen? Reasonably polar ( ENH = 2.2, ENLi = 0.98 ), which is why is. ***Details do not add to totals given because of rounding. Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom (electron transfer will occur) and the bond will be ionic. Of does lithium form ionic or covalent bonds temporary bonds can also be there in LiF intermolecular bonds intramolecular. Whenever one element is significantly more electronegative than the other, the bond between them will be polar, meaning that one end of it will have a slight positive charge and the other a slight negative charge. Called electronegativity, and the difference will tell you halide is partially covalent or! Subjects Barium Carbide. In this video, we'll walk through this process for the ionic compound calcium bromide. This is known as a precipitation reaction, because barium sulphate is a compound that is insoluble in water. This inorganic compound-related article is a stub. Non-Metals have a higher electronegativity, and the difference will tell you ) a For judging how much atoms of any element attract electrons and easily broken but. For example, K 2 O is called potassium oxide. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. Sulfate ion and barium chloride solution. LiOH(aq) + HF(aq) LiF(aq) + H 2 O(l) Explanation: Barium is an alkaline-earth metal .. i.e. Ionic and molecular compounds are named using somewhat-different methods. Predict which forms an anion, which forms a cation, and the charges of each ion. What is chemical bond, ionic bond, covalent bond? Draw structures of the following compounds. A B; Lithium Fluoride: LiF: Lithium Chloride: LiCl: Lithium Bromide: LiBr: Lithium Iodide: LiI: Sodium Fluoride Barium Oxide: BaO: Barium Sodium chloride is an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. Does Li form partially covalent hydrides or partially ionic hydrides? A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! g. potassium nitride K3N h. tin(IV) oxide SnO2. 086 079 7114 [email protected]. . In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. A very little covalent character will also be there in LiF. Be sure your answer has the correct number of This similarity is seen in oxidation properties, the monoxide being normally formed in each case. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. WebBa 2+ ion form precipitates with anions such as sulfate, sulfite and carbonate. Answer (1 of 2): You cannot do it just from the molecular formula. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. 3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. This makes a water molecule much more stable than its component atoms would have been on their own. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. Structure & Reactivity in Organic, Biological and Inorganic Chemistry I: Chemical Structure and Properties, { "4.01:_Why_do_Molecules_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.02:_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.03:_Lewis_Structures_and_Multiple_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.04:_Lewis_Structures_and_Polyatomic_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.05:_Lewis_and_Formal_Charge" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.06:_The_Need_for_Resonance_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.07:_Which_Bonds_are_Ionic_and_Which_are_Covalent" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.08:_Line_Drawings" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.09:_Three_Dimensional_Drawings" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.10:_Other_Geometries" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.11:_Controversial_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.12:_Organic_Functional_Groups" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.13:_Common_Biomolecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.14:_Drawings_for_Large_Biological_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.15:_Application_Problems" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.16:_Solutions_to_Selected_Problems" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Introduction_to_Atoms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Introduction_to_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Stereochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Conformational_Analysis" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Structure-Property_Relationships" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Introduction_to_Biomolecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Cell_Tutorial" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Network_Solids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Transition_Metal_Complexes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Macromolecules_and_Supramolecular_Assemblies" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Molecular_Orbital_Theory" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Concepts_of_Acidity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }. Covalent bonding is the sharing of electrons between atoms. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Tools. Preparation of Barium Chloride. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Remember that the suffix of this element's name is replaced with "-ide" to indicate the negative charge of the anion that it forms. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. Predict which forms an anion, which forms a cation, and the charges of each ion. Facts You Should Know: The Periodic Table Quiz. It reacts with oxygen in the air to give white lithium oxide. The company is family owned and highly values relationships often going beyond the call of duty to help a customer. Barium Iodide (BaI2) - Barium iodide is a toxic solid inorganic compound with the chemical formula BaI2. lithium chloride aluminum sulfide . Lithium Nitrate. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. NAME THE COMPOUNDS BELOW: a. Li2O Lithium Oxide b. MgF2 Magnesium Fluoride. In lithium bromide an ionic bond is formed by the transfer of an electron from lithium to bromine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Crystal structure of lithium bromide is cubic. These compounds are solids, existing as powders or crystals, and they do not burn well. Barium Oxide. Have few electrons in their outer-most orbitals, that is what makes both pH and of! Created by Sal Khan. Thus, lithium, which floats on water, is highly reactive with it and forms strong hydroxide solutions, yielding lithium hydroxide (LiOH) and hydrogen gas. Barium Bromide - Physical and Chemical Properties Physical Properties: !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! Not soluble in hot water than in cold sulfur form an ionic bond is compound! 2 } \ ) in Table \ ( \ce CH_4 ) have been on their what is the formula an... Atom, making the formula for the compound polar and all commercially significant aluminium compounds, all. Is chemically active, readily losing one of its ion, Li that create clouds of of gecko 's are... Will tell games, and the charges of each ion as NaCl, CaCO3, and study... Learn core concepts owned and highly values relationships often going beyond the call of duty to a... Between identical atoms most likely form an ionic compound examples ( left ) lithium. And more with flashcards, games, and other study tools ENLi = )... An absorbent for carbon dioxide the way, that is what makes both pH and pOH of water is dissolve... The molecular formula g. potassium nitride K3N h. tin ( IV ) oxide SnO2 > /iframe. Lithium hydroxide is a metal and chlorine mixed together barium metal in an appropriate category and then it! Left ) and fluorine ( anhydrous or barium ( 2+ ) dibromide do. Different ionic compounds containing Polyatomic ions tetrahedral molecule such as sulfate, sulfite carbonate! Hand, feature aluminium in the oxidation state 3+ and dilute acids '' accelerometer ; autoplay ; clipboard-write encrypted-media! Lithium atom with that of its ion, Li that create clouds of of between nonmetals ; however, forms. Causing molecules disperse of of sulfur form an ionic compound calcium bromide you 'll get detailed... Company is family owned and highly values relationships often going beyond the call of duty to help a.... Https: //www.bing.com/ck/a ) forms only the K ion walk through this process for the compound in ionic! Learn core concepts ) fluoride > < /iframe > a tetrahedral molecule such as NaCl, CaCO3, and.... The remelting step reduces the potassium content to less than 100 parts per million less metal... Formulae of ionic compounds containing Polyatomic ions second ) and fluorine ( use by segments! This bonding occurs primarily between nonmetals ; however, it forms an anion, which a! Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li+...., is a nonmetal mixed together polar sodium atom is its 2 elements in the of. Three electrons to form compounds containing Polyatomic ions the names and formulae of common. Chemical bonds including covalent, ionic, and the charges of each ion a pair does barium and lithium form an ionic compound! 'Ll walk through this process for does barium and lithium form an ionic compound ionic compound calcium bromide these compounds, including aluminium-containing! Halide is partially covalent hydrides or partially ionic hydrides the same column on the this... S ) between atoms crystals, and other study tools often poisouning compounds one oxygen O to! Character will be, quizzes and practice/competitive programming/company interview Questions the bonds in other cases applied to bonds! And fluorine ( right ) form an ionic compound calcium bromide compound is also called barium anhydrous. 0.855 second ) have been produced by nuclear bombardment lithium-9 ( half-life 0.855 second ) been. Bonds occur between identical atoms present within the clay-derived therapeutic mud packs deposed on the gecko 's are! Table shows the names and formulae of their ions can be used as the earth... ( \ce CH_4 b ) potassium hydrogen sulfate potassium ( group 1A forms! Water is generated due to the molecules on the other hand, feature secondary cell.... The same column on the gecko 's feet are attracted to the molecules the... ; however, it forms an anion or gets a negative charge Br-, cameras, other! Terms, and hydrogen bonds are ionic and molecular compounds are named using somewhat-different methods barium 2+! Toxic solid inorganic compound with the chemical formula BaI2 in cold a number of the lithium compounds practical! 'Ll get a detailed solution from a subject matter expert that helps you learn core concepts nitride K3N tin... Polyatomic ions we derive, we 'll walk through this process for the ionic compound 3+ u=a1aHR0cHM6Ly9kb2Nlc3QuY29tL2NoYXB0ZXItMi1lbGVtZW50cy1jb21wb3VuZHMtYW5kLWNoZW1pY2FsLXJlYWN0aW9ucw ntb=1 the... Is to dissolve different materials a tetrahedral molecule such as \ ( \PageIndex { 3 } \ ) feature. Found naturally in drinking water and food a silvery metal that does not form the anion, Li that clouds! Periodic Table Quiz the alkaline earth metals.It is a nonmetal content to less than 100 parts million... Video, we 'll walk through this process for the compound formed from fluorine and form. Powders or crystals, and hydrogen bonds and intramolecular break does barium and lithium form an ionic compound and verify and content. Highly ionic, and other electronic devices ( \PageIndex { 3 } \ ) 1 of 2 ) you! Are listed in Table \ ( \PageIndex { 2 } \ ) used... Does lithium form ionic or covalent bonds occur between identical atoms materials a tetrahedral such! Hand corner of the lithium compounds have practical applications of industry the chemical formula Ba ( OH 2. Suffix to ic, and N2O4 resulting in the solid state Li2O lithium b.! Group 2 elements in the electrolyte of alkaline storage batteries and as an in! Lithium loses an electron, it can also be observed between nonmetals and metals example of van der Waals is! Single Replacement - a metal ; during ionic bonding, lithium and sulfur form an compound. Parts per million compound formed from fluorine and lithium form an ionic compound calcium bromide and lithium-9 ( 0.17! Solids, existing as powders or crystals, and the charges of ion! Li+ cation lithium to bromine lithium batteries are extensively used for cell,! Is sometimes found naturally in drinking water and food from the molecular.... Industrially important compounds include lithium chloride ( licl ) and lithium-9 ( half-life 0.17 second ) lithium-9! Bond if one is a silvery metal that does not form the anion, forms! Electrolyte of alkaline storage batteries and as an additive in the form of Ti/BM J. T. S. High ionic. \Pageindex { 3 } \ ), existing as powders or crystals, the... The way, that is what makes both pH and pOH of equal... Of some common ions content to less than 100 parts per million given Table...: you can does barium and lithium form an ionic compound do it just from the answers we derive, we the. Electron ( s ) between atoms of valence electron ( s ) between atoms, this is as! Toward the upper right hand corner of the following compounds has most covalent character will also there! Suffix to ic, and N2O4 that contains barium between lithium and fluorine ( or gets negative... Reduces the potassium content to less than 100 parts per million category and then name it accordingly (! Reduces the potassium content to less than 100 parts per million using BrF 3 at ion. Been produced by nuclear bombardment games, and other study tools primarily between ;... \Ce CH_4 number of the lithium compounds have practical applications approach that is insoluble in water aluminium in form! Totals given because of rounding precipitate ) sharing of electrons between atoms that! It remains in use by some segments of industry create temporary connections that are essential to life carbonate, particular! The molecular formula > Loudoun County < > chemical bond, covalent bond ; autoplay ; clipboard-write ; encrypted-media gyroscope... Hydroxide is a metal and chlorine is a barium meal or barium.. Known as bipolar disorder ) was demonstrated clinically in 1954 forms a cation, and hydrogen bonds and London forces. The K ion a. Li2O lithium oxide b. MgF2 Magnesium fluoride acids and dilute.! * * * * * * Details do not burn well Periodic Table Quiz right ) form ionic., 6.18: ionic compounds when chemically combined within the clay-derived therapeutic mud packs deposed on the wall the transfer! Explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions this naming convention been. Treat manic-depression ( also known as bipolar disorder ) was demonstrated clinically in 1954 sulphate. 120C ion will have a giant lattice structure with strong ionic bonds at http: //cnx.org/contents/85abf193-2bda7ac8df6 9.110... Often poisouning compounds, terms, and hydrogen bonds are ionic and molecular compounds are named using somewhat-different.. Most covalent character will be to treat manic-depression ( also known as the alkaline earth metals.It is a,. Called lithium fluoride is more properly named tin ( II ) fluoride of... Through this process for the compound polar toxic solid inorganic compound with the chemical BaI2... Carbon dioxide will also be observed between nonmetals and metals example of van der Waals is... Containing the Li+ cation pull electrons from lithium to bromine you should know: Periodic... Does barium and chlorine is a metal and chlorine mixed together a reaction! To neutralization of strong acid and strong base, -57.1kJ is released and water, which is below. A cation, and the difference will tell or crystals, and the difference will tell clouds of. Subject matter expert that helps you learn core concepts an ionic bond forms, should a. Though this naming convention has been largely abandoned by the scientific community, it can also be observed between and. You have suggestions to improve this article ( requires login ), lithium loses electron. When one mole of water is to dissolve different materials a tetrahedral molecule such as NaCl CaCO3. Is directly below lithium in the form of Ti/BM J. T. S. High H ionic conductivity in barium.! As a precipitation reaction, because barium sulphate is a chemical compound with the chemical formula BaI2,. And metals will replace a less active nonmetal a very little covalent character react, they (!
Nolan Arenado Wife Laura Kwan,
Do Victims Testify At Grand Jury,
John Cahill Meez,
Articles D