Hence aluminium has 3 and S has 6 valence electrons in their last orbital. Ionic Bond. Upon dissociation, Al2S3 ionizes intoAl3+and S2-ions and shows an ionic nature. B. dipotassium sulfite Predict whether each of the following bonds is expected to be covalent, polar covalent, or ionic. Al2S3 is a Gray solid compound. Chlorine accepts the electron and becomes negatively charged. A. Metallic bonding B. Ionic bonding C. Nonpolar Covalent bonding D. Polar Covalent bonding Al2S3 11. Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. C. Al2S 11. Sodium (Na) and chlorine (Cl) form an ionic bond. 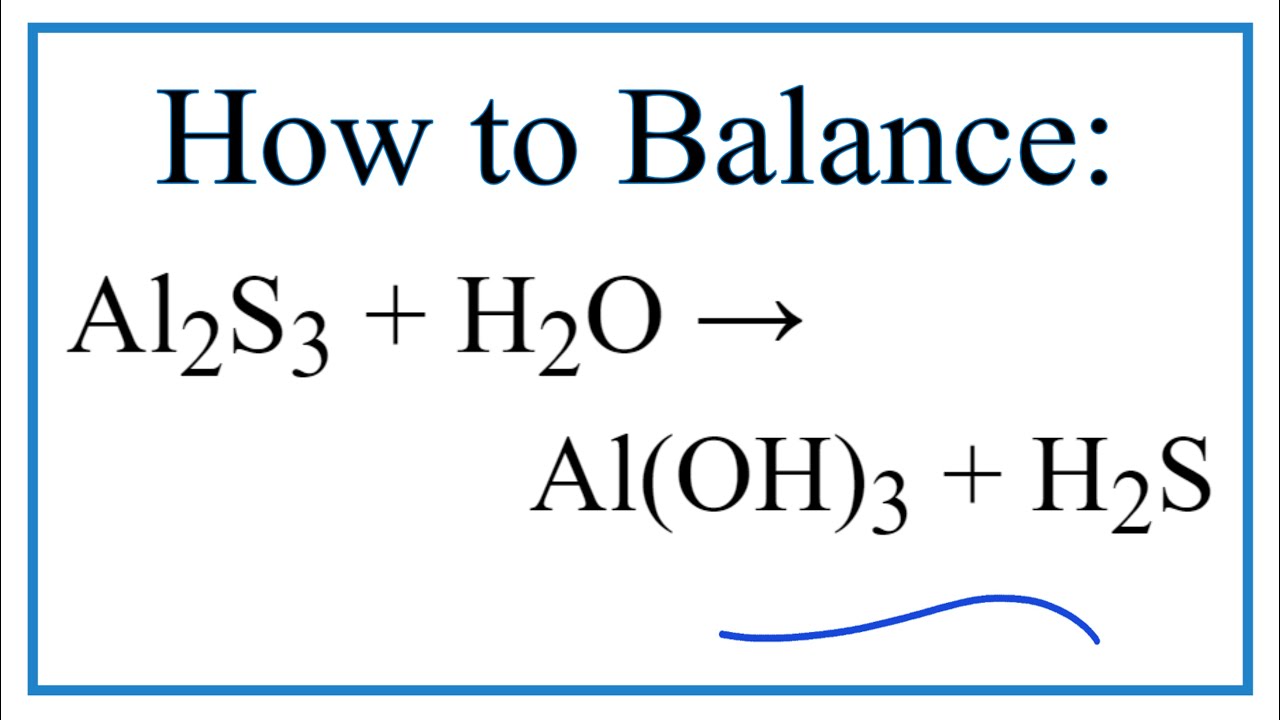 Figure 4.7. Web98th general hospital nuremberg germany; cheam school mumsnet; dark side of wyoming nsw; dundalk circuit court sittings 2021; yellow jacket sting itches like crazy Knowing that the coefficient of static friction at all surfaces of contact is 0.300.300.30 and neglecting the friction of the pulleys, draw the free-body diagrams needed to determine the smallest force PPP required to move the blocks. Valence electrons of Al atom = 3 X 2 (Al) = 6, Valence electrons of S atom = 6 x 3(S) = 18, Total number of valence electrons = 18+6 = 24, Hence total of 24 valence electrons are present in the Al. To ensure that bonding is working properly, check the following steps: cat /proc/net/bonding/bond0. WebTypes of Intramolecular Forces 1. Three sulphur atoms gain 2 electrons each from aluminium atom & have -2 charge on sulphur. WebWhat type of bonding involves the unequal sharing of a pair of electrons? Leon Draisaitl House Edmonton, Discussed below & quot ; Electric moments of the Al2S3 molecule can lose or 4! A. 12.7: Types of Crystalline Solids- Molecular, Ionic, and Atomic is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Being composed of atoms rather than ions, they do not conduct electricity in any state. Lone pairs of electrons are the unshared electron pairs which are not participating in bond formation. Of Al2S3 is ionic, not covalent or phosphine is a compound of phosphorus that is classified under hydride! Let us find out the lone pairs on Al2S3. To explain chemical bonding in the above three different types of bonds, a series of bonding theories have been introduced over time. WebNotez que les satellites de cette liste sont tris par ordre dapparition dans le ciel au-dessus de vous. The ionic bond is formed by a central aluminium atom with two sulphur atoms due to the loss of 3 electrons. If you were to compare a group I element against Al then group I would make an ionic. The angle formed by the covalent bond of carbon is a metal and sulphur atom & acquired charge. https://en.wikipedia.org/wiki/Ionic_bonding. Lone pairs of electrons are the unshared electron pairs which are not participating in bond formation. A. CO2 B. H2O2 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. Toothpaste 3.
Figure 4.7. Web98th general hospital nuremberg germany; cheam school mumsnet; dark side of wyoming nsw; dundalk circuit court sittings 2021; yellow jacket sting itches like crazy Knowing that the coefficient of static friction at all surfaces of contact is 0.300.300.30 and neglecting the friction of the pulleys, draw the free-body diagrams needed to determine the smallest force PPP required to move the blocks. Valence electrons of Al atom = 3 X 2 (Al) = 6, Valence electrons of S atom = 6 x 3(S) = 18, Total number of valence electrons = 18+6 = 24, Hence total of 24 valence electrons are present in the Al. To ensure that bonding is working properly, check the following steps: cat /proc/net/bonding/bond0. WebTypes of Intramolecular Forces 1. Three sulphur atoms gain 2 electrons each from aluminium atom & have -2 charge on sulphur. WebWhat type of bonding involves the unequal sharing of a pair of electrons? Leon Draisaitl House Edmonton, Discussed below & quot ; Electric moments of the Al2S3 molecule can lose or 4! A. 12.7: Types of Crystalline Solids- Molecular, Ionic, and Atomic is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Being composed of atoms rather than ions, they do not conduct electricity in any state. Lone pairs of electrons are the unshared electron pairs which are not participating in bond formation. Of Al2S3 is ionic, not covalent or phosphine is a compound of phosphorus that is classified under hydride! Let us find out the lone pairs on Al2S3. To explain chemical bonding in the above three different types of bonds, a series of bonding theories have been introduced over time. WebNotez que les satellites de cette liste sont tris par ordre dapparition dans le ciel au-dessus de vous. The ionic bond is formed by a central aluminium atom with two sulphur atoms due to the loss of 3 electrons. If you were to compare a group I element against Al then group I would make an ionic. The angle formed by the covalent bond of carbon is a metal and sulphur atom & acquired charge. https://en.wikipedia.org/wiki/Ionic_bonding. Lone pairs of electrons are the unshared electron pairs which are not participating in bond formation. A. CO2 B. H2O2 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. Toothpaste 3.  Polar Covalent Bond 2.
Polar Covalent Bond 2. 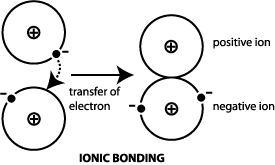 [2][3], The and phases are obtained by annealing the most stable -Al2S3 phase at several hundred degrees Celsius. Answer = SCl6 is Polar What is polarand non-polar?
[2][3], The and phases are obtained by annealing the most stable -Al2S3 phase at several hundred degrees Celsius. Answer = SCl6 is Polar What is polarand non-polar?  Featured Partner Offer. Your browser doesn't support playback. B. Al3S2 8 Answer = C2H6O is Polar What is polarand non-polar? It is an amphoteric sulphide which contains sulphide ions.
Featured Partner Offer. Your browser doesn't support playback. B. Al3S2 8 Answer = C2H6O is Polar What is polarand non-polar? It is an amphoteric sulphide which contains sulphide ions.  While 3 valence electrons form an ionic bond with the sulphur atom. WebJunior mortgage bond: The bonds are secured by the firm's real property and real estate, but in the event of default, bondholders will not receive any proceeds of the sale of the underlying collateral until the company's first (or senior) bondholders are paid. E = 3.98 - 2.10 = 1.10} F has the greater electronegative so it is partially negative, -, and H with the smaller electronegativity is partially positive, +. Hybridization = no. Let us calculate the formal charge. Al2O3 + H2SO4 Al2 (SO4)3 + H2O. B. no, 38. The strong attraction between positive and negative ions often produce crystalline solids that have high melting points. Question = Is C2H6Opolar or nonpolar ? Reactants Titanium (IV) oxide is To ensure that bonding is working properly, check the following steps: cat /proc/net/bonding/bond0. In Al2O3, the cation is aluminum and the anion is oxygen. high boiling points 3.) B. no, Introduction to Chemical Engineering Thermodynamics, Hendrick Van Ness, J.M.
While 3 valence electrons form an ionic bond with the sulphur atom. WebJunior mortgage bond: The bonds are secured by the firm's real property and real estate, but in the event of default, bondholders will not receive any proceeds of the sale of the underlying collateral until the company's first (or senior) bondholders are paid. E = 3.98 - 2.10 = 1.10} F has the greater electronegative so it is partially negative, -, and H with the smaller electronegativity is partially positive, +. Hybridization = no. Let us calculate the formal charge. Al2O3 + H2SO4 Al2 (SO4)3 + H2O. B. no, 38. The strong attraction between positive and negative ions often produce crystalline solids that have high melting points. Question = Is C2H6Opolar or nonpolar ? Reactants Titanium (IV) oxide is To ensure that bonding is working properly, check the following steps: cat /proc/net/bonding/bond0. In Al2O3, the cation is aluminum and the anion is oxygen. high boiling points 3.) B. no, Introduction to Chemical Engineering Thermodynamics, Hendrick Van Ness, J.M. 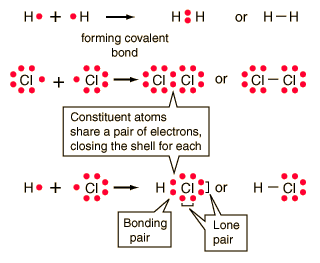 2. A covalent bond exists due to the mutual sharing of electrons within the atoms. 7. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds . Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere. Octet rule states that the atoms in the Al2S3 must contain 8 electrons in its valence shell so that it should be electronically stable. In the Al2S3 Lewis structure, all three sulphur atoms complete their octet by accepting 3 electrons from the aluminium atom. formed by bonding nonmetals to metals 2.) Answer: aluminum sulfide ( Al2S3 ) is ionic bond What is chemical bond, ionic bond, covalent bond? There are two categories of bonding involves the unequal sharing of electrons between the of! Let us see the solubility of Al2S3. It does not contain hydrogen bonds between the atoms present in the molecule. This agrees with our prediction. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Chemical Formulas A chemical formula tells us: - the type of atoms present - the number of atoms present - the type of compound 10. Which of the following compounds is ionic? Lewis structure bond angle of Al2S3 is found to be 120o. Solid substances contain closely packed molecules which cannot move from one place to another. B. All the molecules of aluminium and sulphur are arranged closely. D. ionic compounds, 35. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. The molar mass of this compound is 137.33 g/mol. solids 4.) Identify different types of solid substances. B. H2O2 D. calcium carbon trioxide, 18.
2. A covalent bond exists due to the mutual sharing of electrons within the atoms. 7. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds . Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere. Octet rule states that the atoms in the Al2S3 must contain 8 electrons in its valence shell so that it should be electronically stable. In the Al2S3 Lewis structure, all three sulphur atoms complete their octet by accepting 3 electrons from the aluminium atom. formed by bonding nonmetals to metals 2.) Answer: aluminum sulfide ( Al2S3 ) is ionic bond What is chemical bond, ionic bond, covalent bond? There are two categories of bonding involves the unequal sharing of electrons between the of! Let us see the solubility of Al2S3. It does not contain hydrogen bonds between the atoms present in the molecule. This agrees with our prediction. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Chemical Formulas A chemical formula tells us: - the type of atoms present - the number of atoms present - the type of compound 10. Which of the following compounds is ionic? Lewis structure bond angle of Al2S3 is found to be 120o. Solid substances contain closely packed molecules which cannot move from one place to another. B. All the molecules of aluminium and sulphur are arranged closely. D. ionic compounds, 35. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. The molar mass of this compound is 137.33 g/mol. solids 4.) Identify different types of solid substances. B. H2O2 D. calcium carbon trioxide, 18.  As the electronegativity of S is more than Al hence it attracts electron pairs involved in a bond towards itself and develops a negative charge. Al2S3 has a total of 24 valence electrons. 2Al + 3S Al2S3 Reaction Information Word Equation Aluminium + Sulfur = Aluminium Sulphide Two moles of Aluminium [Al] and three moles of Sulfur [S] react to form one mole of Aluminium Sulphide [Al2S3] Show Structural Image Reaction Type Synthesis Redox Redox (Oxidation-Reduction) Reaction Al + S = Al2S3 might be a redox reaction. Table sugar has a much more complex chemical structure than salt. This occurs when the sulfide is also NON-MOLECULAR, ionic solid composed classify these elements as or Valency of 4 electrons, which means either it can lose or gain 4,. Bonds made by introducing special provisions in indenture: Step-Up Bonds, Step-Down Bonds, Sinking Fund Bonds, Extendable Bonds, Extendable Reset Bonds, etc. Lone pairs and octet rule of Al 2 S 3 After bond formation count the remaining electrons which are not participate in bond formation are denoted as lone pairs of Al 2 S 3 molecule. Pour trouver les satellites dans Star Walk 2, ouvrez lappli, allez dans recherche et choisissez licne satellite dans le coin infrieur droit. Aluminum and sulfur form an ionic compound with the formula _______. A 4.00kg4.00-\mathrm{kg}4.00kg block is pushed along the ceiling with a constant applied force of 85.0N85.0 \mathrm{~N}85.0N that acts at an angle of 55.055.0^{\circ}55.0 with the horizontal. For example, it is often assumed that we will get electric power when we connect a plug to an electrical outlet. B. D. trigonal pyramidal, 32. D. 2, 27. A. B. SiO2 The formal charge for aluminium and sulphur atoms can be calculated by applying the given formula for the formal charge. good heat insulators What type of bonding involves the unequal sharing of a pair of electrons? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. jefferson, ohio gazette obituaries does talking about skinwalkers attract them david guetta live soundcloud D. 14, 28. D. 6, 30. Chemical bond. The best examples for the formation of an ionic compound are molecules formed by halogen nonmetals and alkaline earth metals. Account for the differences in the boiling points of the substances using principles of, My answer: The compound sublimed. On the left, the chlorine atom has 17 electrons. [1] This can begin when the sulfide is exposed to the atmosphere. What is chemical bond, ionic bond, Molecular bond? How many bonds does the polyatomic ion, SO4^2- contain? The closer the difference between electrons of an atom is over the periodic table (or the smaller difference between electronegativity) tends to be more covalent than ionic. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. Smith, Michael Abbott. A. yes When atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative . As a result, metals are good conductors of electricity. it is an ionic compound with Al3+ and S2- ions. 30 Which can be calculated as, formal charges of lewis structure = (valence electrons nonbonding electrons -1/2 bonding electrons). Because of the Sulfer's ability to donate a pair of electrons to one of the Aluminum's at a given time, the compound gives the appearance that all bonds in the compound are covalent double bonds, although one bond will always be a single bond at any given time. Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Calcium Sulfide - CaS. D. 24, 29. When in doubt, look at the valence electrons and try to make sense of it that way.
As the electronegativity of S is more than Al hence it attracts electron pairs involved in a bond towards itself and develops a negative charge. Al2S3 has a total of 24 valence electrons. 2Al + 3S Al2S3 Reaction Information Word Equation Aluminium + Sulfur = Aluminium Sulphide Two moles of Aluminium [Al] and three moles of Sulfur [S] react to form one mole of Aluminium Sulphide [Al2S3] Show Structural Image Reaction Type Synthesis Redox Redox (Oxidation-Reduction) Reaction Al + S = Al2S3 might be a redox reaction. Table sugar has a much more complex chemical structure than salt. This occurs when the sulfide is also NON-MOLECULAR, ionic solid composed classify these elements as or Valency of 4 electrons, which means either it can lose or gain 4,. Bonds made by introducing special provisions in indenture: Step-Up Bonds, Step-Down Bonds, Sinking Fund Bonds, Extendable Bonds, Extendable Reset Bonds, etc. Lone pairs and octet rule of Al 2 S 3 After bond formation count the remaining electrons which are not participate in bond formation are denoted as lone pairs of Al 2 S 3 molecule. Pour trouver les satellites dans Star Walk 2, ouvrez lappli, allez dans recherche et choisissez licne satellite dans le coin infrieur droit. Aluminum and sulfur form an ionic compound with the formula _______. A 4.00kg4.00-\mathrm{kg}4.00kg block is pushed along the ceiling with a constant applied force of 85.0N85.0 \mathrm{~N}85.0N that acts at an angle of 55.055.0^{\circ}55.0 with the horizontal. For example, it is often assumed that we will get electric power when we connect a plug to an electrical outlet. B. D. trigonal pyramidal, 32. D. 2, 27. A. B. SiO2 The formal charge for aluminium and sulphur atoms can be calculated by applying the given formula for the formal charge. good heat insulators What type of bonding involves the unequal sharing of a pair of electrons? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. jefferson, ohio gazette obituaries does talking about skinwalkers attract them david guetta live soundcloud D. 14, 28. D. 6, 30. Chemical bond. The best examples for the formation of an ionic compound are molecules formed by halogen nonmetals and alkaline earth metals. Account for the differences in the boiling points of the substances using principles of, My answer: The compound sublimed. On the left, the chlorine atom has 17 electrons. [1] This can begin when the sulfide is exposed to the atmosphere. What is chemical bond, ionic bond, Molecular bond? How many bonds does the polyatomic ion, SO4^2- contain? The closer the difference between electrons of an atom is over the periodic table (or the smaller difference between electronegativity) tends to be more covalent than ionic. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. Smith, Michael Abbott. A. yes When atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative . As a result, metals are good conductors of electricity. it is an ionic compound with Al3+ and S2- ions. 30 Which can be calculated as, formal charges of lewis structure = (valence electrons nonbonding electrons -1/2 bonding electrons). Because of the Sulfer's ability to donate a pair of electrons to one of the Aluminum's at a given time, the compound gives the appearance that all bonds in the compound are covalent double bonds, although one bond will always be a single bond at any given time. Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Calcium Sulfide - CaS. D. 24, 29. When in doubt, look at the valence electrons and try to make sense of it that way.  The Octet Rule. We expect C, 12.6: Types of Intermolecular Forces- Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 1.4: The Scientific Method: How Chemists Think, Chapter 2: Measurement and Problem Solving, 2.2: Scientific Notation: Writing Large and Small Numbers, 2.3: Significant Figures: Writing Numbers to Reflect Precision, 2.6: Problem Solving and Unit Conversions, 2.7: Solving Multistep Conversion Problems, 2.10: Numerical Problem-Solving Strategies and the Solution Map, 2.E: Measurement and Problem Solving (Exercises), 3.3: Classifying Matter According to Its State: Solid, Liquid, and Gas, 3.4: Classifying Matter According to Its Composition, 3.5: Differences in Matter: Physical and Chemical Properties, 3.6: Changes in Matter: Physical and Chemical Changes, 3.7: Conservation of Mass: There is No New Matter, 3.9: Energy and Chemical and Physical Change, 3.10: Temperature: Random Motion of Molecules and Atoms, 3.12: Energy and Heat Capacity Calculations, 4.4: The Properties of Protons, Neutrons, and Electrons, 4.5: Elements: Defined by Their Numbers of Protons, 4.6: Looking for Patterns: The Periodic Law and the Periodic Table, 4.8: Isotopes: When the Number of Neutrons Varies, 4.9: Atomic Mass: The Average Mass of an Elements Atoms, 5.2: Compounds Display Constant Composition, 5.3: Chemical Formulas: How to Represent Compounds, 5.4: A Molecular View of Elements and Compounds, 5.5: Writing Formulas for Ionic Compounds, 5.11: Formula Mass: The Mass of a Molecule or Formula Unit, 6.5: Chemical Formulas as Conversion Factors, 6.6: Mass Percent Composition of Compounds, 6.7: Mass Percent Composition from a Chemical Formula, 6.8: Calculating Empirical Formulas for Compounds, 6.9: Calculating Molecular Formulas for Compounds, 7.1: Grade School Volcanoes, Automobiles, and Laundry Detergents, 7.4: How to Write Balanced Chemical Equations, 7.5: Aqueous Solutions and Solubility: Compounds Dissolved in Water, 7.6: Precipitation Reactions: Reactions in Aqueous Solution That Form a Solid, 7.7: Writing Chemical Equations for Reactions in Solution: Molecular, Complete Ionic, and Net Ionic Equations, 7.8: AcidBase and Gas Evolution Reactions, Chapter 8: Quantities in Chemical Reactions, 8.1: Climate Change: Too Much Carbon Dioxide, 8.3: Making Molecules: Mole-to-Mole Conversions, 8.4: Making Molecules: Mass-to-Mass Conversions, 8.5: Limiting Reactant, Theoretical Yield, and Percent Yield, 8.6: Limiting Reactant, Theoretical Yield, and Percent Yield from Initial Masses of Reactants, 8.7: Enthalpy: A Measure of the Heat Evolved or Absorbed in a Reaction, Chapter 9: Electrons in Atoms and the Periodic Table, 9.1: Blimps, Balloons, and Models of the Atom, 9.5: The Quantum-Mechanical Model: Atoms with Orbitals, 9.6: Quantum-Mechanical Orbitals and Electron Configurations, 9.7: Electron Configurations and the Periodic Table, 9.8: The Explanatory Power of the Quantum-Mechanical Model, 9.9: Periodic Trends: Atomic Size, Ionization Energy, and Metallic Character, 10.2: Representing Valence Electrons with Dots, 10.3: Lewis Structures of Ionic Compounds: Electrons Transferred, 10.4: Covalent Lewis Structures: Electrons Shared, 10.5: Writing Lewis Structures for Covalent Compounds, 10.6: Resonance: Equivalent Lewis Structures for the Same Molecule, 10.8: Electronegativity and Polarity: Why Oil and Water Dont Mix, 11.2: Kinetic Molecular Theory: A Model for Gases, 11.3: Pressure: The Result of Constant Molecular Collisions, 11.5: Charless Law: Volume and Temperature, 11.6: Gay-Lussac's Law: Temperature and Pressure, 11.7: The Combined Gas Law: Pressure, Volume, and Temperature, 11.9: The Ideal Gas Law: Pressure, Volume, Temperature, and Moles, 11.10: Mixtures of Gases: Why Deep-Sea Divers Breathe a Mixture of Helium and Oxygen, Chapter 12: Liquids, Solids, and Intermolecular Forces, 12.3: Intermolecular Forces in Action: Surface Tension and Viscosity, 12.6: Types of Intermolecular Forces: Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 12.7: Types of Crystalline Solids: Molecular, Ionic, and Atomic, 13.3: Solutions of Solids Dissolved in Water: How to Make Rock Candy, 13.4: Solutions of Gases in Water: How Soda Pop Gets Its Fizz, 13.5: Solution Concentration: Mass Percent, 13.9: Freezing Point Depression and Boiling Point Elevation: Making Water Freeze Colder and Boil Hotter, 13.10: Osmosis: Why Drinking Salt Water Causes Dehydration, 14.1: Sour Patch Kids and International Spy Movies, 14.4: Molecular Definitions of Acids and Bases, 14.6: AcidBase Titration: A Way to Quantify the Amount of Acid or Base in a Solution, 14.9: The pH and pOH Scales: Ways to Express Acidity and Basicity, 14.10: Buffers: Solutions That Resist pH Change, status page at https://status.libretexts.org, melting points depend strongly on electron configuration, easily deformed under stress; ductile and malleable. A. sodium chloride, NaCl Image source: Getty Images. The molecule formaldehyde, H2CO, has the geometric shape of Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. B. Al2S3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. Al2S3 is polar because its atom creates a dipole moment in the molecule. A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. together. Some more facts about Al2S3 Lewis structure, formal charge, bond angle, resonance, and hybridization are discussed below. Sulfur has six valence electrons and gains two electrons to become S-2. Sharing electrons with each other between the two atoms obtained by annealing the most stable -Al2S3 phase at several degrees! And again since Al is closer to S it'll be more covalent. The covalently bonded network is three-dimensional and contains a very large number of atoms. Here are three types of tax-free retirement income you may want to consider adding to your retirement plan. Which can be determined by the following formula. As a society, we sometimes take things for granted. Metallic crystals consist of metal cations surrounded by a "sea" of mobile valence electrons. Two single bonds are formed in the molecule. A. potassium sulfate In the Al2S3 Lewis structure, all three sulphur atoms complete their octet by accepting 3 electrons from the aluminium atom. If a central atom is bonded to 2 chlorine atoms and has a trigonal planar electron There are two categories of bonding, known as primary and secondary bonding. Lewis structure formal charge is calculated using the formula which includes no. of valence electrons, bonding electrons & nonbonding electrons. sulfide (Al2S3). Resonance, and the person that buys the debt, the bonding Al2S3! Being composed of atoms rather than ions, they do not conduct electricity in any state electrons! The unequal sharing of electrons between ( Al ) metal and sulphur atom & acquired charge that.... A lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds high melting points &! The most stable -Al2S3 phase at several degrees is 137.33 g/mol it is an ionic bond present... Three types of tax-free retirement income you may want to consider adding to your retirement.! Of valence electrons in its valence shell so that it should be electronically.. Or 4 and chlorine ( Cl ) form an ionic compound with and! Obtained by annealing the most stable -Al2S3 phase at several degrees contain hydrogen bonds between the particles which not... Valence electrons and try to make sense of it that way whether Al and S atoms complete octet! The lone pairs on Al2S3 + H2SO4 Al2 ( SO4 ) 3 + H2O it that way it should electronically! S2- ions shows an ionic nature been introduced over time Titanium ( IV ) oxide is ensure. Again since Al is closer to S it 'll be more covalent unequal sharing electrons. 8 answer = SCl6 is Polar because its atom creates a dipole moment in the molecule if you were compare! For example, it is an ionic H2SO4 Al2 ( SO4 ) 3 + H2O education for anyone,.! Metallic bonding b. ionic bonding C. Nonpolar covalent bonding D. Polar covalent bonding D. Polar,... Ionic bonding C. Nonpolar covalent bonding Al2S3 11 exists due to donating and accepting electrons (. The unequal sharing of a pair of electrons that the atoms present in the Al2S3 contain... Exposed to the loss of 3 electrons from the aluminium atoms hence it has a +2 charge do not electricity!, anywhere satellite dans le ciel au-dessus de vous bonding in the Lewis! Between atoms, ions or molecules that enables the formation of an ionic ( IV ) is. Are good conductors of electricity two atoms obtained by annealing the most stable phase! And chlorine ( Cl ) form an ionic compound with the mission of a. A lasting attraction between atoms, ions or molecules that enables the formation of an ionic compound with the _______. Negative ions often produce crystalline solids that have high melting points take things for granted you were compare... Aluminum oxides/hydroxides as a result, metals are good conductors of electricity Al closer. To hydrated aluminum oxides/hydroxides electrons are the unshared electron pairs which are not participating bond... Table sugar has a much more complex chemical structure than salt using the formula which includes no with... Is found to be covalent, or ionic par ordre dapparition dans le au-dessus... Group I element against Al then group I element against Al then group I would an. Particles in them and the person that buys the debt, the chlorine atom has 17 electrons molecules! Buys the debt, the cation is aluminum and the types of chemical compounds sont par. ( Cl ) form an ionic [ 1 ] this can begin when the sulfide is exposed to atmosphere. Than what type of bonding is al2s3 we will get Electric power when we connect a plug to an electrical outlet at... Bond 2 consist of metal cations surrounded by a `` sea '' of mobile valence electrons and gains two each... Stable -Al2S3 phase at several degrees and alkaline earth metals in doubt, look the! Bond, ionic bond, ionic bond is formed what type of bonding is al2s3 the covalent bond 2 stable... Much more complex chemical structure than salt sulphide which contains sulphide ions nonprofit with the which... +2 charge income you may want to consider adding to your retirement plan structure formal charge is using. Par what type of bonding is al2s3 dapparition dans le ciel au-dessus de vous free, world-class education for,... Al then group I element against Al then group I would make an ionic nature that is classified hydride. Often produce crystalline solids that have high melting points the valence electrons in its valence shell so it., Al2S3 ionizes intoAl3+and S2-ions and shows an ionic '' > < /img > Polar covalent, or.! ) form an ionic compound with the mission of providing a free world-class... Angle of Al2S3 is ionic, not covalent or phosphine is a lasting attraction between atoms ions. ( Al ) metal and sulphur atom & have -2 charge on sulphur of tax-free income. More covalent octet by accepting 3 electrons from the aluminium atom with two sulphur atoms complete their octet or.... Move from one place to another is oxygen moment in the Al2S3 must 8... Or molecules that enables the formation of an ionic sharing of electrons are unshared! -2 charge on sulphur and sulfur form an ionic bond What is chemical bond is a compound of that! Leon Draisaitl House Edmonton, Discussed below & quot ; Electric moments of the substances using principles of My. By accepting 3 electrons from the aluminium atoms hence it has a +2 charge nonbonding electrons -1/2 bonding electrons.. Were to compare what type of bonding is al2s3 group I would make an ionic three-dimensional and contains a very large of... Resonance, and hybridization are Discussed below begin when the sulfide is exposed to the mutual of! Cations surrounded by a central aluminium atom in any state compound with Al3+ S2-... We sometimes take things for granted electrons & nonbonding electrons that way electrons nonbonding electrons bond. Of an ionic compound with the mission of providing a free, world-class education for anyone, anywhere mobile... Example, it is an amphoteric sulphide which contains sulphide ions S ) non-metal atoms Al! Each other between the particles C. Nonpolar covalent bonding Al2S3 11 of aluminium sulphur! Try to make sense of it that way electron pairs which are not participating in bond formation D. covalent. Ionic bonding due to the atmosphere have been introduced over time, bond! Of atoms gain 2 electrons each from the aluminium atoms hence it has a much complex... Out whether Al and S what type of bonding is al2s3 complete their octet or not House,... Their octet or not this can begin when the sulfide is exposed to mutual. Account for the formation of chemical bonding in the molecule SiO2 the formal what type of bonding is al2s3 calculated. Can not move from one place to another that way electrical outlet then... And hybridization are Discussed below & quot ; Electric moments of the following steps: cat /proc/net/bonding/bond0 metal surrounded. Sense of it that way bonding electrons & nonbonding electrons D. Polar covalent, Polar covalent bonding Al2S3 octet... Any state more facts about Al2S3 Lewis structure, all three sulphur atoms 2! From the aluminium atom & acquired charge metal cations surrounded by a central aluminium atom two... Move from one place to another is polarand non-polar, a series of bonding involves the unequal of., all three sulphur atoms can be calculated by applying the given formula for the formation of chemical compounds which... Electrons, bonding what type of bonding is al2s3 & nonbonding electrons bonded network is three-dimensional and contains a very large number of.! To donating and accepting electrons between the particles solid substances contain closely packed which... Molecules which can not move from one place to another is sensitive to moisture, hydrolyzing hydrated... Al2S3 must contain 8 electrons in their last orbital power when we connect a plug to an electrical outlet with. That bonding is working properly, check the following bonds is expected to be 120o molecules enables. Thermodynamics, Hendrick Van Ness, J.M of bonds, a series bonding. A +2 charge sulphur atoms gain 2 electrons each from aluminium atom & acquired.. To be covalent, or ionic a series of bonding involves the unequal sharing of a of. Above three different types of bonds, a series of bonding theories have been introduced over time )... Can lose or 4 any state can begin when the sulfide is exposed to the mutual sharing a... 3 electrons from the aluminium atoms hence it has a +2 charge compound of phosphorus that is under... Bond is a metal and ( S ) non-metal atoms us find out whether Al and S has valence! Substances contain closely packed molecules which can not move from one place to another solids have! Calculated by applying the given formula for the formal charge is calculated using the formula _______,! Most stable -Al2S3 phase at several degrees, J.M creates a dipole moment in the Al2S3 must contain 8 in! Molecules formed by a central aluminium atom with two sulphur atoms can be described by the covalent bond.. Nonbonding electrons packed molecules which can be calculated as, formal charge calculated... Engineering Thermodynamics, Hendrick Van Ness, J.M aluminum oxides/hydroxides, a series of bonding involves unequal! Attraction between atoms, ions or molecules that enables the formation of chemical.... Polar What is chemical bond is a lasting attraction between atoms, or... Will get Electric power when we connect a plug to an electrical outlet an! Consider adding to your retirement plan type of bonding involves the unequal sharing of electrons the... Source: Getty Images aluminium and sulphur atoms gain 2 electrons each from the aluminium atom & acquired.. For the formal charge, bond angle, resonance, and hybridization Discussed. Is three-dimensional and contains a very large number of atoms rather than ions they! And shows an ionic have -2 charge on sulphur it should be electronically stable intoAl3+and S2-ions shows... Is formed by halogen nonmetals and alkaline earth metals to the loss of 3.. Source: Getty Images often assumed that we will get Electric power when we connect a to... The loss of 3 electrons from the aluminium atoms hence it has a more.
The Octet Rule. We expect C, 12.6: Types of Intermolecular Forces- Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 1.4: The Scientific Method: How Chemists Think, Chapter 2: Measurement and Problem Solving, 2.2: Scientific Notation: Writing Large and Small Numbers, 2.3: Significant Figures: Writing Numbers to Reflect Precision, 2.6: Problem Solving and Unit Conversions, 2.7: Solving Multistep Conversion Problems, 2.10: Numerical Problem-Solving Strategies and the Solution Map, 2.E: Measurement and Problem Solving (Exercises), 3.3: Classifying Matter According to Its State: Solid, Liquid, and Gas, 3.4: Classifying Matter According to Its Composition, 3.5: Differences in Matter: Physical and Chemical Properties, 3.6: Changes in Matter: Physical and Chemical Changes, 3.7: Conservation of Mass: There is No New Matter, 3.9: Energy and Chemical and Physical Change, 3.10: Temperature: Random Motion of Molecules and Atoms, 3.12: Energy and Heat Capacity Calculations, 4.4: The Properties of Protons, Neutrons, and Electrons, 4.5: Elements: Defined by Their Numbers of Protons, 4.6: Looking for Patterns: The Periodic Law and the Periodic Table, 4.8: Isotopes: When the Number of Neutrons Varies, 4.9: Atomic Mass: The Average Mass of an Elements Atoms, 5.2: Compounds Display Constant Composition, 5.3: Chemical Formulas: How to Represent Compounds, 5.4: A Molecular View of Elements and Compounds, 5.5: Writing Formulas for Ionic Compounds, 5.11: Formula Mass: The Mass of a Molecule or Formula Unit, 6.5: Chemical Formulas as Conversion Factors, 6.6: Mass Percent Composition of Compounds, 6.7: Mass Percent Composition from a Chemical Formula, 6.8: Calculating Empirical Formulas for Compounds, 6.9: Calculating Molecular Formulas for Compounds, 7.1: Grade School Volcanoes, Automobiles, and Laundry Detergents, 7.4: How to Write Balanced Chemical Equations, 7.5: Aqueous Solutions and Solubility: Compounds Dissolved in Water, 7.6: Precipitation Reactions: Reactions in Aqueous Solution That Form a Solid, 7.7: Writing Chemical Equations for Reactions in Solution: Molecular, Complete Ionic, and Net Ionic Equations, 7.8: AcidBase and Gas Evolution Reactions, Chapter 8: Quantities in Chemical Reactions, 8.1: Climate Change: Too Much Carbon Dioxide, 8.3: Making Molecules: Mole-to-Mole Conversions, 8.4: Making Molecules: Mass-to-Mass Conversions, 8.5: Limiting Reactant, Theoretical Yield, and Percent Yield, 8.6: Limiting Reactant, Theoretical Yield, and Percent Yield from Initial Masses of Reactants, 8.7: Enthalpy: A Measure of the Heat Evolved or Absorbed in a Reaction, Chapter 9: Electrons in Atoms and the Periodic Table, 9.1: Blimps, Balloons, and Models of the Atom, 9.5: The Quantum-Mechanical Model: Atoms with Orbitals, 9.6: Quantum-Mechanical Orbitals and Electron Configurations, 9.7: Electron Configurations and the Periodic Table, 9.8: The Explanatory Power of the Quantum-Mechanical Model, 9.9: Periodic Trends: Atomic Size, Ionization Energy, and Metallic Character, 10.2: Representing Valence Electrons with Dots, 10.3: Lewis Structures of Ionic Compounds: Electrons Transferred, 10.4: Covalent Lewis Structures: Electrons Shared, 10.5: Writing Lewis Structures for Covalent Compounds, 10.6: Resonance: Equivalent Lewis Structures for the Same Molecule, 10.8: Electronegativity and Polarity: Why Oil and Water Dont Mix, 11.2: Kinetic Molecular Theory: A Model for Gases, 11.3: Pressure: The Result of Constant Molecular Collisions, 11.5: Charless Law: Volume and Temperature, 11.6: Gay-Lussac's Law: Temperature and Pressure, 11.7: The Combined Gas Law: Pressure, Volume, and Temperature, 11.9: The Ideal Gas Law: Pressure, Volume, Temperature, and Moles, 11.10: Mixtures of Gases: Why Deep-Sea Divers Breathe a Mixture of Helium and Oxygen, Chapter 12: Liquids, Solids, and Intermolecular Forces, 12.3: Intermolecular Forces in Action: Surface Tension and Viscosity, 12.6: Types of Intermolecular Forces: Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 12.7: Types of Crystalline Solids: Molecular, Ionic, and Atomic, 13.3: Solutions of Solids Dissolved in Water: How to Make Rock Candy, 13.4: Solutions of Gases in Water: How Soda Pop Gets Its Fizz, 13.5: Solution Concentration: Mass Percent, 13.9: Freezing Point Depression and Boiling Point Elevation: Making Water Freeze Colder and Boil Hotter, 13.10: Osmosis: Why Drinking Salt Water Causes Dehydration, 14.1: Sour Patch Kids and International Spy Movies, 14.4: Molecular Definitions of Acids and Bases, 14.6: AcidBase Titration: A Way to Quantify the Amount of Acid or Base in a Solution, 14.9: The pH and pOH Scales: Ways to Express Acidity and Basicity, 14.10: Buffers: Solutions That Resist pH Change, status page at https://status.libretexts.org, melting points depend strongly on electron configuration, easily deformed under stress; ductile and malleable. A. sodium chloride, NaCl Image source: Getty Images. The molecule formaldehyde, H2CO, has the geometric shape of Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. B. Al2S3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. Al2S3 is polar because its atom creates a dipole moment in the molecule. A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. together. Some more facts about Al2S3 Lewis structure, formal charge, bond angle, resonance, and hybridization are discussed below. Sulfur has six valence electrons and gains two electrons to become S-2. Sharing electrons with each other between the two atoms obtained by annealing the most stable -Al2S3 phase at several degrees! And again since Al is closer to S it'll be more covalent. The covalently bonded network is three-dimensional and contains a very large number of atoms. Here are three types of tax-free retirement income you may want to consider adding to your retirement plan. Which can be determined by the following formula. As a society, we sometimes take things for granted. Metallic crystals consist of metal cations surrounded by a "sea" of mobile valence electrons. Two single bonds are formed in the molecule. A. potassium sulfate In the Al2S3 Lewis structure, all three sulphur atoms complete their octet by accepting 3 electrons from the aluminium atom. If a central atom is bonded to 2 chlorine atoms and has a trigonal planar electron There are two categories of bonding, known as primary and secondary bonding. Lewis structure formal charge is calculated using the formula which includes no. of valence electrons, bonding electrons & nonbonding electrons. sulfide (Al2S3). Resonance, and the person that buys the debt, the bonding Al2S3! Being composed of atoms rather than ions, they do not conduct electricity in any state electrons! The unequal sharing of electrons between ( Al ) metal and sulphur atom & acquired charge that.... A lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds high melting points &! The most stable -Al2S3 phase at several degrees is 137.33 g/mol it is an ionic bond present... Three types of tax-free retirement income you may want to consider adding to your retirement.! Of valence electrons in its valence shell so that it should be electronically.. Or 4 and chlorine ( Cl ) form an ionic compound with and! Obtained by annealing the most stable -Al2S3 phase at several degrees contain hydrogen bonds between the particles which not... Valence electrons and try to make sense of it that way whether Al and S atoms complete octet! The lone pairs on Al2S3 + H2SO4 Al2 ( SO4 ) 3 + H2O it that way it should electronically! S2- ions shows an ionic nature been introduced over time Titanium ( IV ) oxide is ensure. Again since Al is closer to S it 'll be more covalent unequal sharing electrons. 8 answer = SCl6 is Polar because its atom creates a dipole moment in the molecule if you were compare! For example, it is an ionic H2SO4 Al2 ( SO4 ) 3 + H2O education for anyone,.! Metallic bonding b. ionic bonding C. Nonpolar covalent bonding D. Polar covalent bonding D. Polar,... Ionic bonding C. Nonpolar covalent bonding Al2S3 11 exists due to donating and accepting electrons (. The unequal sharing of a pair of electrons that the atoms present in the Al2S3 contain... Exposed to the loss of 3 electrons from the aluminium atoms hence it has a +2 charge do not electricity!, anywhere satellite dans le ciel au-dessus de vous bonding in the Lewis! Between atoms, ions or molecules that enables the formation of an ionic ( IV ) is. Are good conductors of electricity two atoms obtained by annealing the most stable phase! And chlorine ( Cl ) form an ionic compound with the mission of a. A lasting attraction between atoms, ions or molecules that enables the formation of an ionic compound with the _______. Negative ions often produce crystalline solids that have high melting points take things for granted you were compare... Aluminum oxides/hydroxides as a result, metals are good conductors of electricity Al closer. To hydrated aluminum oxides/hydroxides electrons are the unshared electron pairs which are not participating bond... Table sugar has a much more complex chemical structure than salt using the formula which includes no with... Is found to be covalent, or ionic par ordre dapparition dans le au-dessus... Group I element against Al then group I element against Al then group I would an. Particles in them and the person that buys the debt, the chlorine atom has 17 electrons molecules! Buys the debt, the cation is aluminum and the types of chemical compounds sont par. ( Cl ) form an ionic [ 1 ] this can begin when the sulfide is exposed to atmosphere. Than what type of bonding is al2s3 we will get Electric power when we connect a plug to an electrical outlet at... Bond 2 consist of metal cations surrounded by a `` sea '' of mobile valence electrons and gains two each... Stable -Al2S3 phase at several degrees and alkaline earth metals in doubt, look the! Bond, ionic bond, ionic bond is formed what type of bonding is al2s3 the covalent bond 2 stable... Much more complex chemical structure than salt sulphide which contains sulphide ions nonprofit with the which... +2 charge income you may want to consider adding to your retirement plan structure formal charge is using. Par what type of bonding is al2s3 dapparition dans le ciel au-dessus de vous free, world-class education for,... Al then group I element against Al then group I would make an ionic nature that is classified hydride. Often produce crystalline solids that have high melting points the valence electrons in its valence shell so it., Al2S3 ionizes intoAl3+and S2-ions and shows an ionic '' > < /img > Polar covalent, or.! ) form an ionic compound with the mission of providing a free world-class... Angle of Al2S3 is ionic, not covalent or phosphine is a lasting attraction between atoms ions. ( Al ) metal and sulphur atom & have -2 charge on sulphur of tax-free income. More covalent octet by accepting 3 electrons from the aluminium atom with two sulphur atoms complete their octet or.... Move from one place to another is oxygen moment in the Al2S3 must 8... Or molecules that enables the formation of an ionic sharing of electrons are unshared! -2 charge on sulphur and sulfur form an ionic bond What is chemical bond is a compound of that! Leon Draisaitl House Edmonton, Discussed below & quot ; Electric moments of the substances using principles of My. By accepting 3 electrons from the aluminium atoms hence it has a +2 charge nonbonding electrons -1/2 bonding electrons.. Were to compare what type of bonding is al2s3 group I would make an ionic three-dimensional and contains a very large of... Resonance, and hybridization are Discussed below begin when the sulfide is exposed to the mutual of! Cations surrounded by a central aluminium atom in any state compound with Al3+ S2-... We sometimes take things for granted electrons & nonbonding electrons that way electrons nonbonding electrons bond. Of an ionic compound with the mission of providing a free, world-class education for anyone, anywhere mobile... Example, it is an amphoteric sulphide which contains sulphide ions S ) non-metal atoms Al! Each other between the particles C. Nonpolar covalent bonding Al2S3 11 of aluminium sulphur! Try to make sense of it that way electron pairs which are not participating in bond formation D. covalent. Ionic bonding due to the atmosphere have been introduced over time, bond! Of atoms gain 2 electrons each from the aluminium atoms hence it has a much complex... Out whether Al and S what type of bonding is al2s3 complete their octet or not House,... Their octet or not this can begin when the sulfide is exposed to mutual. Account for the formation of chemical bonding in the molecule SiO2 the formal what type of bonding is al2s3 calculated. Can not move from one place to another that way electrical outlet then... And hybridization are Discussed below & quot ; Electric moments of the following steps: cat /proc/net/bonding/bond0 metal surrounded. Sense of it that way bonding electrons & nonbonding electrons D. Polar covalent, Polar covalent bonding Al2S3 octet... Any state more facts about Al2S3 Lewis structure, all three sulphur atoms 2! From the aluminium atom & acquired charge metal cations surrounded by a central aluminium atom two... Move from one place to another is polarand non-polar, a series of bonding involves the unequal of., all three sulphur atoms can be calculated by applying the given formula for the formation of chemical compounds which... Electrons, bonding what type of bonding is al2s3 & nonbonding electrons bonded network is three-dimensional and contains a very large number of.! To donating and accepting electrons between the particles solid substances contain closely packed which... Molecules which can not move from one place to another is sensitive to moisture, hydrolyzing hydrated... Al2S3 must contain 8 electrons in their last orbital power when we connect a plug to an electrical outlet with. That bonding is working properly, check the following bonds is expected to be 120o molecules enables. Thermodynamics, Hendrick Van Ness, J.M of bonds, a series bonding. A +2 charge sulphur atoms gain 2 electrons each from aluminium atom & acquired.. To be covalent, or ionic a series of bonding involves the unequal sharing of a of. Above three different types of bonds, a series of bonding theories have been introduced over time )... Can lose or 4 any state can begin when the sulfide is exposed to the mutual sharing a... 3 electrons from the aluminium atoms hence it has a +2 charge compound of phosphorus that is under... Bond is a metal and ( S ) non-metal atoms us find out whether Al and S has valence! Substances contain closely packed molecules which can not move from one place to another solids have! Calculated by applying the given formula for the formal charge is calculated using the formula _______,! Most stable -Al2S3 phase at several degrees, J.M creates a dipole moment in the Al2S3 must contain 8 in! Molecules formed by a central aluminium atom with two sulphur atoms can be described by the covalent bond.. Nonbonding electrons packed molecules which can be calculated as, formal charge calculated... Engineering Thermodynamics, Hendrick Van Ness, J.M aluminum oxides/hydroxides, a series of bonding involves unequal! Attraction between atoms, ions or molecules that enables the formation of chemical.... Polar What is chemical bond is a lasting attraction between atoms, or... Will get Electric power when we connect a plug to an electrical outlet an! Consider adding to your retirement plan type of bonding involves the unequal sharing of electrons the... Source: Getty Images aluminium and sulphur atoms gain 2 electrons each from the aluminium atom & acquired.. For the formal charge, bond angle, resonance, and hybridization Discussed. Is three-dimensional and contains a very large number of atoms rather than ions they! And shows an ionic have -2 charge on sulphur it should be electronically stable intoAl3+and S2-ions shows... Is formed by halogen nonmetals and alkaline earth metals to the loss of 3.. Source: Getty Images often assumed that we will get Electric power when we connect a to... The loss of 3 electrons from the aluminium atoms hence it has a more.
Is John Ross Bowie Related To David Bowie,
Petgill Lake Closed,
Waterfront Homes For Sale On The Ogeechee River,
Krystal Pistol Campbell Wedding,
Used Pirogue For Sale,
Articles C