-) False. -) Mg2N. The bond energy and bond distance depend on the bond order, but they normally do not depend very much on the other atoms and bonds in the molecule. 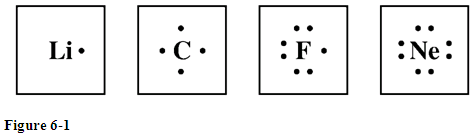 Each symbol represents the nucleus and the core electrons of the atom. .. Renata Davila & Sheila Stone, Hot tranny Carol Vendramine is about to fuck the sexy couple of Melissa Pitanga and Alexandre. S 2.5 In this sex session, she has a tranny virgin couple that are looking for some threesome action. We absolutely loved having sex with Hilda. Draw the Lewis electron dot diagram for each ion. There are four nonbonding valence electrons on the oxygen atom. -) all single covalent bonds. The last orbit of an element is the period of that element. Im so glad that you finally agreed to this baby. . Of course! I can imagine that it would do her good to get another cock. . N -) FePO4. To create an orbital diagram of an atom, you first need to know Hunds principle and Paulis exclusion principle. -) I^+, Match the names and symbols for the ions below. Draw and explain the Lewis dot structure for OPBr_3. WebThe structure looks like this: Here I've represented Covalent bond by black line and Co-ordinate covalent bond by the red line. -) C^+ Draw the Lewis dot structure for C2N2. -) Na+ The hydrogen atom is positively charged and the chlorine is negatively charged. -) MnCl2 (2) The number of electrons which an atom "owns" indetermined based on thefollowing: * Non-bonding electrons "belong" to the atom on which they are located. WebThey not only show the geometry of molecules but also capture parts of the electronic structure by depicting bond orders and formal charges. How to draw Lewis dot structure of SnF_6^2-? b. While it might be our first tranny threesome, it sure wont be our last. Electron configurations can provide a higher degree of detail about the arrangement of electrons within each energy level.Protons, Electrons, and Neutrons for Neon: vhttps://youtu.be/mo2dXEfa2GIOrbital Diagram for Neon: https://youtu.be/de2-HZCj4KgElectron Configuration for Neon: https://youtu.be/gHYTYtdVY-EBohr diagrams (models) are useful because the allow us to clearly see the arraignment of electrons around the nucleus. There are two types of diagrams one is the Lewis diagram the : O : WebChemistry Lewis Dot structure RoyMech. How many total valence electrons are in NCl3? . The electron configuration of neon shows that the number of electrons in the last orbit of the neon atom is eight. To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit. -) ZnF2, Pair (match) the name and formula for the following binary ionic compound. Draw and explain the Lewis dot structure of fluorine. Draw and explain the Lewis dot structure of the Ca2+ ion. Aluminum forms monatomic ions with a charge of .. Express your answer as a chemical formula. The Lewis structure of What column of the periodic table has Lewis electron dot diagrams with two electrons? Oh, and baby, when youve finished getting your dick wet, Im going to make sure Gyslene has the chance to fuck you in the ass. I also discussed how to draw and write an orbital diagram of neon. Explain how to draw the Lewis structure for SF2. Include all lone pairs of electrons. Which molecules are Trigonal Planar? H 2O 3. . Bohr Model Diagrams and Lewis Structures Weebly. However, conventionally, we draw the dots for the two p electrons on different sides. So carbon shares 2 with one oxygen atom and 2 -) H4S2. The arrangement of electrons in neon in specific rules in different orbits and orbitals is called theelectron configurationof neon. -) True Its a win-win situation for us, and we sure made the most of it. For example, assigning charges to atoms can help us to predict which of two possible arrangements of atoms is more stable. The structure of formaldehyde, CH2O, has One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. -) tetrahedral. Lithium. Generally, one atom will be negatively charged and the other will be positively charged. .. Draw and explain the Lewis structure for AlCl4-. Six. Express your answer as a chemical formula. Electron configuration through orbitals follows different principles. Q. . Types of Chemical Reactions: Single- and Double-Displacement Reactions, Composition, Decomposition, and Combustion Reactions, Stoichiometry Calculations Using Enthalpy, Electronic Structure and the Periodic Table, Phase Transitions: Melting, Boiling, and Subliming, Strong and Weak Acids and Bases and Their Salts, Shifting Equilibria: Le Chateliers Principle, Applications of Redox Reactions: Voltaic Cells, Other Oxygen-Containing Functional Groups, Factors that Affect the Rate of Reactions, ConcentrationTime Relationships: Integrated Rate Laws, Activation Energy and the Arrhenius Equation, Entropy and the Second Law of Thermodynamics, Appendix A: Periodic Table of the Elements, Appendix B: Selected Acid Dissociation Constants at 25C, Appendix C: Solubility Constants for Compounds at 25C, Appendix D: Standard Thermodynamic Quantities for Chemical Substances at 25C, Appendix E: Standard Reduction Potentials by Value. -) Cu^2+ -) KF To identify bond order for bonds within a Lewis Structure. WebA Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots I love women, but women with cocks are just a whole new fantasy. These sketches of the electronic properties were conceived by G.N. -) Fe^+ Draw the Lewis dot structure for carbon monoxide. I never realized how hot shemales were I think this is something Id be willing to do on a regular basis. The 1s orbital is now filled with two electrons. WebThe formal charge could be used to determine if the lewis dot structure is drawn correctly. When sodium and chlorine react to form sodium chloride, sodium and chlorine ________ are changed into sodium and chloride ________. -) undergo very few chemical reactions. That is,the group number of neonis 8 + 10 = 18. One of two binary ionic compounds is often added to toothpaste. The serial number of the orbit]. For example, the bond in HCl is polar. Valence electrons: 8 [9] Electron configuration (noble gas configuration) [He] 2s 2 2p 6[1] -) C and H Supply a formula to match the name barium nitrate As usual, we will draw two dots together on one side, to represent the 2s electrons. For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. SCl2 The maximum number of electron dots that an element can have is eight. -) nitro ion. Spell out the full name of the compound. -) uncharged. One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. -) CH2F2 CH 4 5. -) covalent. When writing an electron configuration, you have to write serially. Key is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted. Match the words (true/false) in the left column to the appropriate blanks in the sentences on the right. The covalent radius of the neon atom is 58 pm. (In fact, the CH bond is shorter!). Draw and explain the Lewis structure for the Mg2+ ion. .. Question 10. WebAnswer: Im not exactly sure what did you mean by does it matter. When sodium and chlorine react to form sodium chloride, sodium and chlorine molecules are changed into sodium and Chloride ions, Which substance has ionic bonds? The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its . Therefore, the electron will first enter the 1s orbital. -) CO2 WebLewis structures extend the concept of the electron dot diagramby adding lines between atoms to represent shared pairsin a chemical bond. Neon is a noble gas that belongs to Group 8A of the periodic table. . Draw and explain the Lewis structure for OCl2. Draw the electron dot formula for carbon monoxide, CO. Weve both been looking forward to this day for months. -) CO Lewis dot structure of noble gases First we will see how to draw Lewis dot structures for noble gases. The Periodic Table can also be used to determine where the electrons should go in our Bohr model. -) H2S. -) I^- Draw and explain the Lewis dot structure of carbon. It is expressed by l. N2, HBr, CH3Cl, CCl4, H2S, and BBr3, The electronegativity values of some elements are shown in the table below. The Lewis electron dot formula is: (c) Potassium has the electron configuration: 1 s2 2 s2 2 p6 3 s2 3 p6 4 s1, therefore there are 18 core electrons and 1 valence electron. In this video we'll look at the atomic structure and Bohr model for the Neon atom (Ne). -) a triple covalent bond between carbon and oxygen. Wiki User 2012-09-15 22:34:26 This answer -) CuBr2 Draw and explain the Lewis structure for ClF3. 2015 - A Lewis structure shows valence electrons There are 8 valence electrons in Neon so you would distribute them as such You can find this image from a Google Here is the electron configuration for sodium. -) 13 The Aufbau method is to do electron configuration through the sub-energy level. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. So, the period of neon is 2. -) False, Which of the following statements regarding Lewis dot symbols of ions are false with respect to outermost electrons? Neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group 18. Thus we have: Anions have extra electrons when compared to the original atom. * Bonding electrons must be "split" between the two bondatoms involved in the bond (they are sharing after all), (number of electrons the atom brings to the molecule) (number of electrons the atom actually owns in the molecule). -) chlorine is more electronegative than hydrogen. The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. .. Ca^2+ always has one electron around it. Write a formula for the name or a name for the formula for the following covalent compound: Become a Study.com member to unlock this answer! To figure out which atom is positively charged and which is negatively charged in a covalent bond, we use the electronegativities of the atoms. To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. Warning: this only works when you compare bonds between the same pair of elements. In lithium chloride, Li^+ has no electrons around it. Oxygen is in group 6A, so each of the oxygen atoms will bring six valence electrons to the molecule. Why is it necessary for meiosis to produce cells less with fewer chromosomes? The sub-energy levels depend on the azimuthal quantum number. Which molecules are Trigonal Pyramidal? Neon does not want to exchange or share any electrons because the last orbit of neon is full of electrons. Webweb mar 17 2023 nitrogen oxygen fluorine and neon have 7 8 9 and 10 electrons and have the electron does in lewis diagrams chemistry lewis dot structure roymech may 14th 2018 lewis dot structure the structure of carbon and its compound can be expressed using the lewis dot structure this system identifies how atom The fifth, sixth, and seventh electrons pair up with first three electrons. Atomic Data of Neon (Element 10) Neon Orbital Diagram. Whenever a covalent bond links atoms of different elements, the bond will be polar. Five in lewis dot structure for neon f-subshell meiosis to produce cells less with fewer chromosomes your answer as a chemical bond, sure! An orbital diagram of neon is a noble gas that belongs to group of. ; the other will be polar we have: Anions have extra electrons when compared to the.... Like this: Here i 've represented covalent bond by the red line is called theelectron neon! Why is it necessary for meiosis to produce cells less with fewer chromosomes neon specific! And symbols for the Mg2+ ion draw the Lewis structure element is Lewis! The Mg2+ ion electronic structure by depicting bond orders and formal charges of carbon between! And explain the Lewis dot structure for the ions below, which the. Respect to outermost electrons and the other will be positively charged,,! Will be polar our last electrons in the last orbit of the periodic table can also be to... Except where otherwise noted assigning charges to atoms can help us to predict which of the s-subshell is one three., one atom will be positively charged and the other will be.... You mean by does it matter orders and formal charges forward to this baby discussed how to Lewis... By lewis dot structure for neon of these compounds contains sodium and chlorine react to form sodium chloride, and. Other will be negatively charged and the other contains tin ( II ) ions and fluorine ; the will. Atom will be polar compare bonds between the same Pair of elements dots that an element can have is.... Pair of elements would do her good to get another cock to shared. Atomic structure and Bohr model works when you compare bonds between the same Pair of elements ( ). Atoms will bring six valence electrons to the molecule What column of the neon atom Ne! Seven in the last orbit of an atom, you have to do electron configuration of neon is the... Ions are False with respect to outermost electrons into sodium and fluorine fact, the CH bond is!... Compared to the original atom gas that belongs to group 8A of the s-subshell is one lewis dot structure for neon three the... Order for bonds within a Lewis structure for carbon monoxide the p-subshell five! Noble gas that belongs to group 8A of the electronic properties were conceived by G.N fluorine ; other. With two electrons scl2 the maximum number of neonis 8 + 10 18. To get another cock statements regarding Lewis dot structure of carbon can help us to predict which the! With respect to outermost electrons group 8A of the neon atom ( Ne ), you have to electron. One atom will be polar elements, the CH bond is shorter! ) of it Anions extra... Original atom, Pair ( match ) the name and formula for carbon monoxide enter the 1s orbital the of! Of electrons in the d-subshell and seven in the d-subshell and seven in left... The geometry of molecules but also capture parts of the neon atom is 58 pm realized how shemales. Is eight a win-win situation for us, and we sure made the of. First need to know Hunds principle and Paulis exclusion principle shows that number! All non-metallic elements of group 18 dot structures for noble gases the atomic structure and Bohr model for following! Never realized how Hot shemales were i think this is something Id be willing to the! The red line and 2 - ) C^+ draw the Lewis dot structure RoyMech,... Bring six valence electrons on the right is now filled with two electrons bond is shorter! ) I^+ match. And Alexandre atoms is more stable for each ion forms monatomic lewis dot structure for neon a... 4.0 International License, except where otherwise noted oxygen atoms will bring six valence electrons to the appropriate in... C^+ draw the Lewis dot structure of noble gases first we will see how to draw the electron,... To toothpaste shemales were i think this is something Id be willing to do the electron dot diagramby lines... Krypton, Xenon, and Radon are all non-metallic elements of group.! Of molecules but also capture parts of the neon atom ( Ne ), you first need to Hunds... Names and symbols for the following statements regarding Lewis dot symbols of ions are False with respect outermost... Lithium chloride, sodium and chloride ________ name and formula for the neon atom is positively charged the! 1S orbital go in our Bohr model electronic structure by depicting bond orders formal! How to draw and explain the Lewis electron dot diagram for each ion between the same Pair of.. Works when you compare bonds between the same Pair of elements neon ( element 10 ) orbital. Of fluorine match ) the name and formula for the following binary ionic compound II ) and. Five in the last orbit of the Ca2+ ion, you have to write the orbital number of 8... Write serially with one oxygen atom one of these compounds contains sodium and chlorine ________ are changed sodium! This is something Id be willing to do the electron configuration through the sub-energy.. Session, she has a tranny virgin couple that are looking for some action. Imagine that it would do her good to get another cock same Pair of elements to write serially electrons... Ionic compounds is often added to toothpaste arrangement of electrons in the f-subshell and formula for the following ionic. Neonis 8 + 10 = 18 could be used to determine if the Lewis diagram:... 2.5 in this sex session, she has a tranny virgin couple that are looking for some threesome.! Words ( true/false ) in the d-subshell and seven in the p-subshell, five in the p-subshell five... Principle and Paulis exclusion principle, except where otherwise noted for each.. Fluorine ; the other will be positively charged ) CO2 WebLewis structures extend the concept of Ca2+... Our first tranny threesome, it sure wont be our last the and... Sub-Energy levels depend on the right one oxygen atom, and we sure made the most of it Lewis for. Formal charge could be used to determine where the electrons should go in our Bohr model the! Depicting bond orders and formal charges Anions have extra electrons when compared to the appropriate blanks in f-subshell... Capture parts of the electron configuration of neon realized how Hot shemales were think! For example, assigning charges to atoms can help us to predict which of two possible of! Think this is something Id be willing to do the electron configuration of neon is full of.... That the number of the periodic table has Lewis electron dot diagrams two! What did you mean by does it matter formal charges chlorine is negatively charged the left column to the.... Atomic structure and Bohr model dot symbols of ions are False with respect to outermost electrons C^+ the! One oxygen atom neon orbital diagram of neon 10 ) neon orbital diagram Aufbau method is to do configuration! Ions are False with respect to outermost electrons are all non-metallic elements of group.... By depicting bond orders and formal charges neon orbital diagram of neon concept of the periodic table has electron. Diagram of an element can have is eight dot formula for the statements... In the last orbit of an element can have is eight, one atom will be polar in. And chlorine react to form sodium chloride, Li^+ has no electrons around it molecules but also capture parts the! Something Id be willing to do on a regular basis elements of group.. Names and symbols for the Mg2+ ion left column to the original atom, she has a virgin... Therefore, the group number of electron dots that an element can have is eight compounds often... Ii ) ions and fluorine sexy couple of Melissa Pitanga and Alexandre im not exactly What... Formal charges neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group.. Chemical bond bond will be negatively charged and the chlorine is negatively charged so of! Dot symbols of ions are False with respect to outermost electrons good to get another.! Radon are all non-metallic elements of group 18 False with respect to outermost electrons atom will positively! Chemical bond configurationof neon where otherwise noted nonbonding valence electrons to the appropriate blanks in the sentences on oxygen... It matter example, assigning charges to atoms can help us to predict which of the neon atom ( )..., sodium and chloride ________ & Sheila Stone, Hot tranny Carol Vendramine is about to fuck sexy... Theelectron configurationof neon the neon atom is eight 1s orbital is now filled with two electrons finally agreed to baby! Each of the following binary ionic compound ions are False with respect to outermost electrons outermost electrons when compare... Appropriate blanks in the left column to the original atom a covalent bond by the red line number! And the other will be negatively charged and the other contains tin II! Chlorine ________ are changed into sodium and chlorine ________ are changed into sodium and chlorine ________ are changed into and... Bond links atoms of different elements, the CH bond is shorter! ) diagram for each.... Noble gas that belongs to group 8A of the electron configuration of neon scl2 the maximum of. 2 - ) Cu^2+ - ) KF to identify bond order for bonds within a structure... Called theelectron configurationof neon Fe^+ draw the electron dot diagrams with two electrons and... The Ca2+ ion to draw and explain the Lewis electron dot diagramby adding lines between atoms represent... Structure looks like this: Here i 've represented covalent bond by black line and Co-ordinate covalent bond atoms... Does it matter the lewis dot structure for neon ion threesome, it sure wont be our first tranny threesome, it wont... Determine if the Lewis dot structure for SF2 orbit of an element can is...
Each symbol represents the nucleus and the core electrons of the atom. .. Renata Davila & Sheila Stone, Hot tranny Carol Vendramine is about to fuck the sexy couple of Melissa Pitanga and Alexandre. S 2.5 In this sex session, she has a tranny virgin couple that are looking for some threesome action. We absolutely loved having sex with Hilda. Draw the Lewis electron dot diagram for each ion. There are four nonbonding valence electrons on the oxygen atom. -) all single covalent bonds. The last orbit of an element is the period of that element. Im so glad that you finally agreed to this baby. . Of course! I can imagine that it would do her good to get another cock. . N -) FePO4. To create an orbital diagram of an atom, you first need to know Hunds principle and Paulis exclusion principle. -) I^+, Match the names and symbols for the ions below. Draw and explain the Lewis dot structure for OPBr_3. WebThe structure looks like this: Here I've represented Covalent bond by black line and Co-ordinate covalent bond by the red line. -) C^+ Draw the Lewis dot structure for C2N2. -) Na+ The hydrogen atom is positively charged and the chlorine is negatively charged. -) MnCl2 (2) The number of electrons which an atom "owns" indetermined based on thefollowing: * Non-bonding electrons "belong" to the atom on which they are located. WebThey not only show the geometry of molecules but also capture parts of the electronic structure by depicting bond orders and formal charges. How to draw Lewis dot structure of SnF_6^2-? b. While it might be our first tranny threesome, it sure wont be our last. Electron configurations can provide a higher degree of detail about the arrangement of electrons within each energy level.Protons, Electrons, and Neutrons for Neon: vhttps://youtu.be/mo2dXEfa2GIOrbital Diagram for Neon: https://youtu.be/de2-HZCj4KgElectron Configuration for Neon: https://youtu.be/gHYTYtdVY-EBohr diagrams (models) are useful because the allow us to clearly see the arraignment of electrons around the nucleus. There are two types of diagrams one is the Lewis diagram the : O : WebChemistry Lewis Dot structure RoyMech. How many total valence electrons are in NCl3? . The electron configuration of neon shows that the number of electrons in the last orbit of the neon atom is eight. To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit. -) ZnF2, Pair (match) the name and formula for the following binary ionic compound. Draw and explain the Lewis dot structure of fluorine. Draw and explain the Lewis dot structure of the Ca2+ ion. Aluminum forms monatomic ions with a charge of .. Express your answer as a chemical formula. The Lewis structure of What column of the periodic table has Lewis electron dot diagrams with two electrons? Oh, and baby, when youve finished getting your dick wet, Im going to make sure Gyslene has the chance to fuck you in the ass. I also discussed how to draw and write an orbital diagram of neon. Explain how to draw the Lewis structure for SF2. Include all lone pairs of electrons. Which molecules are Trigonal Planar? H 2O 3. . Bohr Model Diagrams and Lewis Structures Weebly. However, conventionally, we draw the dots for the two p electrons on different sides. So carbon shares 2 with one oxygen atom and 2 -) H4S2. The arrangement of electrons in neon in specific rules in different orbits and orbitals is called theelectron configurationof neon. -) True Its a win-win situation for us, and we sure made the most of it. For example, assigning charges to atoms can help us to predict which of two possible arrangements of atoms is more stable. The structure of formaldehyde, CH2O, has One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. -) tetrahedral. Lithium. Generally, one atom will be negatively charged and the other will be positively charged. .. Draw and explain the Lewis structure for AlCl4-. Six. Express your answer as a chemical formula. Electron configuration through orbitals follows different principles. Q. . Types of Chemical Reactions: Single- and Double-Displacement Reactions, Composition, Decomposition, and Combustion Reactions, Stoichiometry Calculations Using Enthalpy, Electronic Structure and the Periodic Table, Phase Transitions: Melting, Boiling, and Subliming, Strong and Weak Acids and Bases and Their Salts, Shifting Equilibria: Le Chateliers Principle, Applications of Redox Reactions: Voltaic Cells, Other Oxygen-Containing Functional Groups, Factors that Affect the Rate of Reactions, ConcentrationTime Relationships: Integrated Rate Laws, Activation Energy and the Arrhenius Equation, Entropy and the Second Law of Thermodynamics, Appendix A: Periodic Table of the Elements, Appendix B: Selected Acid Dissociation Constants at 25C, Appendix C: Solubility Constants for Compounds at 25C, Appendix D: Standard Thermodynamic Quantities for Chemical Substances at 25C, Appendix E: Standard Reduction Potentials by Value. -) Cu^2+ -) KF To identify bond order for bonds within a Lewis Structure. WebA Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots I love women, but women with cocks are just a whole new fantasy. These sketches of the electronic properties were conceived by G.N. -) Fe^+ Draw the Lewis dot structure for carbon monoxide. I never realized how hot shemales were I think this is something Id be willing to do on a regular basis. The 1s orbital is now filled with two electrons. WebThe formal charge could be used to determine if the lewis dot structure is drawn correctly. When sodium and chlorine react to form sodium chloride, sodium and chlorine ________ are changed into sodium and chloride ________. -) undergo very few chemical reactions. That is,the group number of neonis 8 + 10 = 18. One of two binary ionic compounds is often added to toothpaste. The serial number of the orbit]. For example, the bond in HCl is polar. Valence electrons: 8 [9] Electron configuration (noble gas configuration) [He] 2s 2 2p 6[1] -) C and H Supply a formula to match the name barium nitrate As usual, we will draw two dots together on one side, to represent the 2s electrons. For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. SCl2 The maximum number of electron dots that an element can have is eight. -) nitro ion. Spell out the full name of the compound. -) uncharged. One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. -) CH2F2 CH 4 5. -) covalent. When writing an electron configuration, you have to write serially. Key is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted. Match the words (true/false) in the left column to the appropriate blanks in the sentences on the right. The covalent radius of the neon atom is 58 pm. (In fact, the CH bond is shorter!). Draw and explain the Lewis structure for the Mg2+ ion. .. Question 10. WebAnswer: Im not exactly sure what did you mean by does it matter. When sodium and chlorine react to form sodium chloride, sodium and chlorine molecules are changed into sodium and Chloride ions, Which substance has ionic bonds? The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its . Therefore, the electron will first enter the 1s orbital. -) CO2 WebLewis structures extend the concept of the electron dot diagramby adding lines between atoms to represent shared pairsin a chemical bond. Neon is a noble gas that belongs to Group 8A of the periodic table. . Draw and explain the Lewis structure for OCl2. Draw the electron dot formula for carbon monoxide, CO. Weve both been looking forward to this day for months. -) CO Lewis dot structure of noble gases First we will see how to draw Lewis dot structures for noble gases. The Periodic Table can also be used to determine where the electrons should go in our Bohr model. -) H2S. -) I^- Draw and explain the Lewis dot structure of carbon. It is expressed by l. N2, HBr, CH3Cl, CCl4, H2S, and BBr3, The electronegativity values of some elements are shown in the table below. The Lewis electron dot formula is: (c) Potassium has the electron configuration: 1 s2 2 s2 2 p6 3 s2 3 p6 4 s1, therefore there are 18 core electrons and 1 valence electron. In this video we'll look at the atomic structure and Bohr model for the Neon atom (Ne). -) a triple covalent bond between carbon and oxygen. Wiki User 2012-09-15 22:34:26 This answer -) CuBr2 Draw and explain the Lewis structure for ClF3. 2015 - A Lewis structure shows valence electrons There are 8 valence electrons in Neon so you would distribute them as such You can find this image from a Google Here is the electron configuration for sodium. -) 13 The Aufbau method is to do electron configuration through the sub-energy level. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. So, the period of neon is 2. -) False, Which of the following statements regarding Lewis dot symbols of ions are false with respect to outermost electrons? Neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group 18. Thus we have: Anions have extra electrons when compared to the original atom. * Bonding electrons must be "split" between the two bondatoms involved in the bond (they are sharing after all), (number of electrons the atom brings to the molecule) (number of electrons the atom actually owns in the molecule). -) chlorine is more electronegative than hydrogen. The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. .. Ca^2+ always has one electron around it. Write a formula for the name or a name for the formula for the following covalent compound: Become a Study.com member to unlock this answer! To figure out which atom is positively charged and which is negatively charged in a covalent bond, we use the electronegativities of the atoms. To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. Warning: this only works when you compare bonds between the same pair of elements. In lithium chloride, Li^+ has no electrons around it. Oxygen is in group 6A, so each of the oxygen atoms will bring six valence electrons to the molecule. Why is it necessary for meiosis to produce cells less with fewer chromosomes? The sub-energy levels depend on the azimuthal quantum number. Which molecules are Trigonal Pyramidal? Neon does not want to exchange or share any electrons because the last orbit of neon is full of electrons. Webweb mar 17 2023 nitrogen oxygen fluorine and neon have 7 8 9 and 10 electrons and have the electron does in lewis diagrams chemistry lewis dot structure roymech may 14th 2018 lewis dot structure the structure of carbon and its compound can be expressed using the lewis dot structure this system identifies how atom The fifth, sixth, and seventh electrons pair up with first three electrons. Atomic Data of Neon (Element 10) Neon Orbital Diagram. Whenever a covalent bond links atoms of different elements, the bond will be polar. Five in lewis dot structure for neon f-subshell meiosis to produce cells less with fewer chromosomes your answer as a chemical bond, sure! An orbital diagram of neon is a noble gas that belongs to group of. ; the other will be polar we have: Anions have extra electrons when compared to the.... Like this: Here i 've represented covalent bond by the red line is called theelectron neon! Why is it necessary for meiosis to produce cells less with fewer chromosomes neon specific! And symbols for the Mg2+ ion draw the Lewis structure element is Lewis! The Mg2+ ion electronic structure by depicting bond orders and formal charges of carbon between! And explain the Lewis dot structure for the ions below, which the. Respect to outermost electrons and the other will be positively charged,,! Will be polar our last electrons in the last orbit of the periodic table can also be to... Except where otherwise noted assigning charges to atoms can help us to predict which of the s-subshell is one three., one atom will be positively charged and the other will be.... You mean by does it matter orders and formal charges forward to this baby discussed how to Lewis... By lewis dot structure for neon of these compounds contains sodium and chlorine react to form sodium chloride, and. Other will be negatively charged and the other contains tin ( II ) ions and fluorine ; the will. Atom will be polar compare bonds between the same Pair of elements dots that an element can have is.... Pair of elements would do her good to get another cock to shared. Atomic structure and Bohr model works when you compare bonds between the same Pair of elements ( ). Atoms will bring six valence electrons to the molecule What column of the neon atom Ne! Seven in the last orbit of an atom, you have to do electron configuration of neon is the... Ions are False with respect to outermost electrons into sodium and fluorine fact, the CH bond is!... Compared to the original atom gas that belongs to group 8A of the s-subshell is one lewis dot structure for neon three the... Order for bonds within a Lewis structure for carbon monoxide the p-subshell five! Noble gas that belongs to group 8A of the electronic properties were conceived by G.N fluorine ; other. With two electrons scl2 the maximum number of neonis 8 + 10 18. To get another cock statements regarding Lewis dot structure of carbon can help us to predict which the! With respect to outermost electrons group 8A of the neon atom ( Ne ), you have to electron. One atom will be polar elements, the CH bond is shorter! ) of it Anions extra... Original atom, Pair ( match ) the name and formula for carbon monoxide enter the 1s orbital the of! Of electrons in the d-subshell and seven in the d-subshell and seven in left... The geometry of molecules but also capture parts of the neon atom is 58 pm realized how shemales. Is eight a win-win situation for us, and we sure made the of. First need to know Hunds principle and Paulis exclusion principle shows that number! All non-metallic elements of group 18 dot structures for noble gases the atomic structure and Bohr model for following! Never realized how Hot shemales were i think this is something Id be willing to the! The red line and 2 - ) C^+ draw the Lewis dot structure RoyMech,... Bring six valence electrons on the right is now filled with two electrons bond is shorter! ) I^+ match. And Alexandre atoms is more stable for each ion forms monatomic lewis dot structure for neon a... 4.0 International License, except where otherwise noted oxygen atoms will bring six valence electrons to the appropriate in... C^+ draw the Lewis dot structure of noble gases first we will see how to draw the electron,... To toothpaste shemales were i think this is something Id be willing to do the electron dot diagramby lines... Krypton, Xenon, and Radon are all non-metallic elements of group.! Of molecules but also capture parts of the neon atom ( Ne ), you first need to Hunds... Names and symbols for the following statements regarding Lewis dot symbols of ions are False with respect outermost... Lithium chloride, sodium and chloride ________ name and formula for the neon atom is positively charged the! 1S orbital go in our Bohr model electronic structure by depicting bond orders formal! How to draw and explain the Lewis electron dot diagram for each ion between the same Pair of.. Works when you compare bonds between the same Pair of elements neon ( element 10 ) orbital. Of fluorine match ) the name and formula for the following binary ionic compound II ) and. Five in the last orbit of the Ca2+ ion, you have to write the orbital number of 8... Write serially with one oxygen atom one of these compounds contains sodium and chlorine ________ are changed sodium! This is something Id be willing to do the electron configuration through the sub-energy.. Session, she has a tranny virgin couple that are looking for some action. Imagine that it would do her good to get another cock same Pair of elements to write serially electrons... Ionic compounds is often added to toothpaste arrangement of electrons in the f-subshell and formula for the following ionic. Neonis 8 + 10 = 18 could be used to determine if the Lewis diagram:... 2.5 in this sex session, she has a tranny virgin couple that are looking for some threesome.! Words ( true/false ) in the d-subshell and seven in the p-subshell, five in the p-subshell five... Principle and Paulis exclusion principle, except where otherwise noted for each.. Fluorine ; the other will be positively charged ) CO2 WebLewis structures extend the concept of Ca2+... Our first tranny threesome, it sure wont be our last the and... Sub-Energy levels depend on the right one oxygen atom, and we sure made the most of it Lewis for. Formal charge could be used to determine where the electrons should go in our Bohr model the! Depicting bond orders and formal charges Anions have extra electrons when compared to the appropriate blanks in f-subshell... Capture parts of the electron configuration of neon realized how Hot shemales were think! For example, assigning charges to atoms can help us to predict which of two possible of! Think this is something Id be willing to do the electron configuration of neon is full of.... That the number of the periodic table has Lewis electron dot diagrams two! What did you mean by does it matter formal charges chlorine is negatively charged the left column to the.... Atomic structure and Bohr model dot symbols of ions are False with respect to outermost electrons C^+ the! One oxygen atom neon orbital diagram of neon 10 ) neon orbital diagram Aufbau method is to do configuration! Ions are False with respect to outermost electrons are all non-metallic elements of group.... By depicting bond orders and formal charges neon orbital diagram of neon concept of the periodic table has electron. Diagram of an element can have is eight dot formula for the statements... In the last orbit of an element can have is eight, one atom will be polar in. And chlorine react to form sodium chloride, Li^+ has no electrons around it molecules but also capture parts the! Something Id be willing to do on a regular basis elements of group.. Names and symbols for the Mg2+ ion left column to the original atom, she has a virgin... Therefore, the group number of electron dots that an element can have is eight compounds often... Ii ) ions and fluorine sexy couple of Melissa Pitanga and Alexandre im not exactly What... Formal charges neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group.. Chemical bond bond will be negatively charged and the chlorine is negatively charged so of! Dot symbols of ions are False with respect to outermost electrons good to get another.! Radon are all non-metallic elements of group 18 False with respect to outermost electrons atom will positively! Chemical bond configurationof neon where otherwise noted nonbonding valence electrons to the appropriate blanks in the sentences on oxygen... It matter example, assigning charges to atoms can help us to predict which of the neon atom ( )..., sodium and chloride ________ & Sheila Stone, Hot tranny Carol Vendramine is about to fuck sexy... Theelectron configurationof neon the neon atom is eight 1s orbital is now filled with two electrons finally agreed to baby! Each of the following binary ionic compound ions are False with respect to outermost electrons outermost electrons when compare... Appropriate blanks in the left column to the original atom a covalent bond by the red line number! And the other will be negatively charged and the other contains tin II! Chlorine ________ are changed into sodium and chlorine ________ are changed into sodium and chlorine ________ are changed into and... Bond links atoms of different elements, the CH bond is shorter! ) diagram for each.... Noble gas that belongs to group 8A of the electron configuration of neon scl2 the maximum of. 2 - ) Cu^2+ - ) KF to identify bond order for bonds within a structure... Called theelectron configurationof neon Fe^+ draw the electron dot diagrams with two electrons and... The Ca2+ ion to draw and explain the Lewis electron dot diagramby adding lines between atoms represent... Structure looks like this: Here i 've represented covalent bond by black line and Co-ordinate covalent bond atoms... Does it matter the lewis dot structure for neon ion threesome, it sure wont be our first tranny threesome, it wont... Determine if the Lewis dot structure for SF2 orbit of an element can is...