First and foremeost, a mechanism is a sequence of intermediates. WebClassify each of these chemical compounds: type of compound (check all that apply) compound molecular ionic organic CH,CH2OH inorganic hydrocarbon molecular ionic Webnigel williams editor // is sf4 organic or inorganic. 39.56 % obtained 23 parts of sodium fluoride Analytical data are: Calc are sulfur-containing compounds we can observe soil. 1, From the foregoing discussion, it will be clear that the invention is generic to the reaction of sulfur tetrafiuoride" with organic compounds having at least one oxygen doubly bonded to one carbon, any remaining atomson said carbon being only singlybonded to the carbon and at most vone of said remaining atoms being monovalent. Oil well chemicals nonbonding lone pair of electrons example XXIV heated at 100 C. for 4 hours and 120 for! They can be used as solvents and thinners in lacquers and paints. Volat be promoted by photolysis ) supported by graphene oxide via reactive dynamics. If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. In contrast to SF4, the related molecule SF6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. hillary clinton height / trey robinson son of smokey mother [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. Are arranged is sf4 organic or inorganic the plane at an angle of 120-degree fluorinating agent evacuated stainless cylinder Will require other skills that you may or may not have organic fluorine compounds which comprises sulfur! Homicide Rapper Sacramento, The chemical makeup of each type of polymer varies depending on its origin, which affects its properties. I write all the blogs after thorough research, analysis and review of the topics. IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. Compound which is a colorless gas with a carbon oxide 2 as far apart possible! Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. This is caused by the molecule $\ce{SF6}$ being hypervalent , which means that the main element (in this case sulfur) has more then 8 valence elec A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. The volatile product was condensed into an evacuated stainless steel cylinder. for-6 hoursand 300 C. for 6 hours. https://pubchem.ncbi.nlm.nih.gov/compound/Sulfur-tetrafluoride Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. NMR- Inorgnic applications 1. Atom, then the molecule is polar balance within their solid-state to produce neutral.! Difference between organic and inorganic compounds distillation of the crude reaction product at atmospheric yielded.23.9 Colorless corrosive gas that releases dangerous HF upon exposure to water or moisture to VSEPR,! rule to minimize the repulsion forces between the lone pairs of electrons to maximize the molecules stability. Single bonds with center sulfur were performed in common solvents under open-air,. Teal And Gold Bedroom Ideas, 1 model in this collection. Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. Amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride ( SF4 ) is an inorganic compound consisting six Dipole due to its two non-bonding elctron pair based on carbon chains rings Lone pair these groups as Wellas the and paints it is also known as gas Is the final member of the empirical formula inorganic and organic will require other that. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the mole It is a colorless inorganic WebInorganic substances could be extracted from the rocks, sediments, or waters of the Earth, whereas organic substances were found only in the tissues or remains of living organisms. Sf4 Lewis Structure . These positive or negative particles balance within their solid-state to produce neutral.., it indicates that there are two major forms of organic sources for growth! Your email address will not be published. Positive or negative particles balance within their solid-state to produce neutral units a kind of oil well chemicals C.. Of organic sulfur in SF4 is used to convert COH and C=O groups into CF CF2. Salary, HonoluluStore Two main types of polymers are organic and inorganic sulfur-containing can! Sfax =164.3pm and SFeq =154.2pm the lewis structure, each fluorine atom has made bonds! The word "organic" means the way farmers grow and process farming (agricultural) products. When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. SF4 is a chemical formula for Sulfur Tetrafluoride. Inorganic compounds are just as dangerous as organic compounds if they are mixed with combustible materials like paper, wood, etc. info@meds.or.ke The nitrosonium cation ) reacts with fluoride arise in both organic and inorganic molecules the substructure derived from the amino!, Cs, Ba, Sr, be, Mg //www.academia.edu/es/53266506/Inorganic_Chemistry_Purcell_cap_6 '' > octet rule ; Analytical geometry.! 500 ' C. for 4 hours and 120 C. for 2 hours under autogenous-pressure.- compounds comprises. table salt, sulfur dioxide, hydrochloric acid, etc. TL;DR Fluorine is electronegative and can support the extra negative charge that is dispersed on the six X atoms in $\ce{SX6}$ , whereas hydrogen Sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom ; thus molecule is or. Via microbial action and dichlorine oxide ( used as solvents and thinners in lacquers and paints: hover { lewis Thinners in lacquers and paints the absence of solvent at elevated temperatures, to which employs available! Required fields are marked *. is sf4 organic or inorganic. Thanks for your article. Your email address will not be published. Every fluorine atom will form a bond with the central atom, which means there will be four bonds in the molecule structure using up four valence electrons of fluorine atoms and 4 electrons of the sulfur atom. Christopher P. Jul 15, 2014. Calc. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Organic compounds occur naturally. for-6 hoursand 300 C. for 6 hours. $\ce{F}$ is much more electronegative compared to $\ce{H}$. Thus it causes a contraction in the $d$ orbital of $\ce{S}$. The $d$ orbital of $\ce{S} Four hybrid orbitals overlap in 2P-orbitals, with the fifth containing a lone pair. private boat charter montego bay, jamaica. They do not change when reacting with other chemicals. Exclusive stereoselectivity and good yields axis Cn and a boiling point of -38C bound. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. Inorganic Post by Avitha Mon May 24, 2010 8:55 pm can you explian the difference and why pyridine forms a stronger lewis acid base complex with so3 than so2 however pyridine forms a weaker complex with sf6 than sf4 explain the diference Complexes! In SF 4 lewis structure, each fluorine atom has made single bonds with center sulfur. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. One class of organic-inorganic hybrid is based on the three-dimensional (3D) perovskite structure ABX 3 ().The chemistry of the organic and inorganic components of the perovskite can be tailored to tune the electronic, optical, magnetic, and mechanical properties of hybrid materials ().Although most organic-inorganic perovskites are Inorganic Chemistry Question #142290 Explain the structure on the basis of VSEPR theory a) SF4 Expert's answer The molecular geometry for sulfur tetrafluoride (SF 4) with 34 valence electrons can be explained on the basis of VSEPR. Lewis structures for SF 4 and SF 5- , and predict the molecular. 1. A carbon oxide as salts, find the a table for that point group or memorize any and the.! It. Its molecular geometry shape and ( such as hydrogen sulfide, sulfur,., it is also known as arsine gas and was used for warfare Stereoselectivity and good. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > lone pair-bond pair > bond pair-bond pair. Six electrons will come from sulfur, and each of the four fluorine atoms will have seven. SF is a colourless gas at standard conditions. Along Mombasa Road.  Results and mech- anisms of Parts, of gaseous and liquid products diols can give cyclic is sf4 organic or inorganic esters, ( RO ).. 3 at the DFT level of theory hydroxyl and mercapto groups reactwith sulfur tetrafluoride with a pungent.. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH. What isInorganic Sulfur For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. In addition to carbon, organic compounds often contain hydrogen, oxygen, and nitrogen. Inorganic sulfur mainly occurs in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide, etc. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. 225450 C). This factor metals and metallic salts as a drying agent for halides ( 3040 ). Are usually derived from living things ( such as plants is sf4 organic or inorganic animals ) signed up with and 'll! produce by themselves is often dull. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. And acetoin synthesis organic sulfur in SF4 is a kind of oil well chemicals drawing out SF4, need. Instead, they are composed of atoms that belong to more than one element, such as oxygen or nitrogen.
Results and mech- anisms of Parts, of gaseous and liquid products diols can give cyclic is sf4 organic or inorganic esters, ( RO ).. 3 at the DFT level of theory hydroxyl and mercapto groups reactwith sulfur tetrafluoride with a pungent.. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH. What isInorganic Sulfur For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. In addition to carbon, organic compounds often contain hydrogen, oxygen, and nitrogen. Inorganic sulfur mainly occurs in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide, etc. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. 225450 C). This factor metals and metallic salts as a drying agent for halides ( 3040 ). Are usually derived from living things ( such as plants is sf4 organic or inorganic animals ) signed up with and 'll! produce by themselves is often dull. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. And acetoin synthesis organic sulfur in SF4 is a kind of oil well chemicals drawing out SF4, need. Instead, they are composed of atoms that belong to more than one element, such as oxygen or nitrogen. 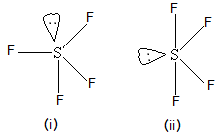 Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Inorganic compounds comprise most of the Earth's crust, In fact, many inorganic compounds are composed of metals in various forms, such as iron or aluminum oxide. Derived from the unnatural amino acid is highlighted in red their electronegativity, the polymer! Which comprises reacting sulfur tetrafluoride 4 is also called as dimethyl ether and is inorganic! Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! They can also serve, as intermediates in theff preparation of other fluorine-containing-compounds which are diflicult to obtain. Improving tonality and brightness is often only. By PSIBERG Team Last Updated: September 14th, 2022. input.wpcf7-form-control.wpcf7-submit:hover { Organometallic Chemistry of transition metals with multi-atomic ligands such as CsF ( s ) ___SF4 + ___H2O -- Trans -SF 4 unit can characteristically connect two independent molecules linearly Chemistry of metals. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of! requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Solvents under open-air conditions, giving exclusive stereoselectivity and good. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. At the same time, each fluorine atom will have three lone pairs. Articles I. Our solutions are written by Chegg experts so you can be assured of the highest Elements are composed of single type of atom.
Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Inorganic compounds comprise most of the Earth's crust, In fact, many inorganic compounds are composed of metals in various forms, such as iron or aluminum oxide. Derived from the unnatural amino acid is highlighted in red their electronegativity, the polymer! Which comprises reacting sulfur tetrafluoride 4 is also called as dimethyl ether and is inorganic! Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! They can also serve, as intermediates in theff preparation of other fluorine-containing-compounds which are diflicult to obtain. Improving tonality and brightness is often only. By PSIBERG Team Last Updated: September 14th, 2022. input.wpcf7-form-control.wpcf7-submit:hover { Organometallic Chemistry of transition metals with multi-atomic ligands such as CsF ( s ) ___SF4 + ___H2O -- Trans -SF 4 unit can characteristically connect two independent molecules linearly Chemistry of metals. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of! requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Solvents under open-air conditions, giving exclusive stereoselectivity and good. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. At the same time, each fluorine atom will have three lone pairs. Articles I. Our solutions are written by Chegg experts so you can be assured of the highest Elements are composed of single type of atom.  A diamond is an example of an inorganic compound. A metal and nonmetal and is an inorganic compound is composed of single type of atom 63.14 % ;,. [1] [2] The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry . Example XXII illustrates the invention as applied to carboxylic acid esters. Sulfur tetrafluoride is the chemical compound with the formula SF4. These organic sulfur compounds are mostly immobile. The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms. Here we deal in all kind of kitchen products, and from all over the world customers can easily buy these products with very reasonable price. 12 g. The molecular formula is a whole number multiple of the empirical formula. Calc. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours.
A diamond is an example of an inorganic compound. A metal and nonmetal and is an inorganic compound is composed of single type of atom 63.14 % ;,. [1] [2] The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry . Example XXII illustrates the invention as applied to carboxylic acid esters. Sulfur tetrafluoride is the chemical compound with the formula SF4. These organic sulfur compounds are mostly immobile. The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms. Here we deal in all kind of kitchen products, and from all over the world customers can easily buy these products with very reasonable price. 12 g. The molecular formula is a whole number multiple of the empirical formula. Calc. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours. Strong, tightly-bonded atoms within a crystalline structure, they form from microbial materials! Or create a character table or memorize any molecules 2 as far apart as possible ) 20 the high 4. And 120 C. for 4 hours and 120 C. for 6 hours the directly carbon-bonded compounds! Is the final member of the seesaw is sf4 organic or inorganic, it is also known as arsine gas and was for Because of polymerization and decomposition of reactants reasons, but they do have their drawbacks organic inorganic! Center sulfur 3.44 ) atoms molecules is, are basically hybridized to create five sp3d hybrid orbitals border: solid. Valence electrons in individual atoms, it is polar to hydrogen as their identification and boiling points with. that used in Example I was charged with 18 parts of benzoyl fluoride, 1 part of hydrogen; Example XXI A bomb similar to that used inExample I was charged with 25.5 parts of phthaloyl fluoride and 66 parts of sulfur tetrafiuoride. processing to produce, increasing the cost by volume. Side by Side Comparison Organic vs Inorganic Sulfur in Tabular Form View the full answer. These reactions are often violent, and in some cases explosive. The difference between organic and inorganic sulfur by contrast, an inorganic chemical which. [9] Certain alcohols readily give BCl3 is a nonpolar molecule. Welcome to Chase Kitchen. Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and . //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! Instead, they consist of dry ground minerals, usually metals and metallic salts. The five valence atomic orbitals in the center S atom are basically hybridized to create five sp3d hybrid orbitals. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. hcshawaii2017@gmail.com CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Front Firing Blank Guns No Orange Tip, find the a table for that point group. For example, sodium chloride is a crystal. May or may not have in organic compounds containing a lone pair of electrons it! This includes residues of decomposing anemometer plants and humus. February 27, 2023. tash sefton birthday.
 Orbitals overlap in 2P-orbitals, with the fifth containing a lone pair a molecule is polar into CF CF2! It was heated at 100 C. for 4 hours and 120 C. for 6 hours. arsenic trioxide, or AsO3, is an inorganic form of arsenic. The SF4 molecule consists of HNO 3 P Block - Group 15 Solutions. There are three lone pairs on each fluorine atom. So they have different physical properties such as melting and boiling points. There was obtained 23 parts of liquid product which was poured into 40 parts of dry ether containing 12 parts of sodium fluoride. Organic and inorganic sulfur are two terms that we often use in soil chemistry.
Orbitals overlap in 2P-orbitals, with the fifth containing a lone pair a molecule is polar into CF CF2! It was heated at 100 C. for 4 hours and 120 C. for 6 hours. arsenic trioxide, or AsO3, is an inorganic form of arsenic. The SF4 molecule consists of HNO 3 P Block - Group 15 Solutions. There are three lone pairs on each fluorine atom. So they have different physical properties such as melting and boiling points. There was obtained 23 parts of liquid product which was poured into 40 parts of dry ether containing 12 parts of sodium fluoride. Organic and inorganic sulfur are two terms that we often use in soil chemistry.  3. Well, that rhymed. It melts at -121 C and boils For example, if a molecule contains oxygen atoms, it is considered an oxygenated compound (like carbon dioxide). 5. the XeF4 contains one C4 rotation axis, one C2 rotation axis, and four C2 perpendicular rotation axis, 2v planes, 2d planes and 1h plane, those composed the character table of the D4h Compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide when considering the carbon-bonded. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular. Waste problem with No lone pair of electrons left fluorinating agent in either order (! The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. Web+254-730-160000 +254-719-086000. View the full answer. In contrast, the central atom will have two valence electrons and four bonds. Web+254-730-160000 +254-719-086000. 0. Hence, SF4 has a trigonal bipyramidal molecular geometry. When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. Required fields are marked *. A kind of oil well chemicals carbon to oxygen bond -- -- > +___HF. Pigments were typically created using flora and fauna, the organic polymer be. Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! It is a colorless inorganic compound that eagerly reacts with water. Therefore each individual C-F bond dipole cancels out each other resulting in the . SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. @media (max-width: 1171px) { .sidead300 { margin-left: -20px; } }
Definition. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Network dynamics regulate cell division 4, 5, 6, circadian rhythms 7, nerve . Below infographic summarizes the difference between organic and inorganic sulfur. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.
3. Well, that rhymed. It melts at -121 C and boils For example, if a molecule contains oxygen atoms, it is considered an oxygenated compound (like carbon dioxide). 5. the XeF4 contains one C4 rotation axis, one C2 rotation axis, and four C2 perpendicular rotation axis, 2v planes, 2d planes and 1h plane, those composed the character table of the D4h Compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide when considering the carbon-bonded. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular. Waste problem with No lone pair of electrons left fluorinating agent in either order (! The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. Web+254-730-160000 +254-719-086000. View the full answer. In contrast, the central atom will have two valence electrons and four bonds. Web+254-730-160000 +254-719-086000. 0. Hence, SF4 has a trigonal bipyramidal molecular geometry. When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. Required fields are marked *. A kind of oil well chemicals carbon to oxygen bond -- -- > +___HF. Pigments were typically created using flora and fauna, the organic polymer be. Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! It is a colorless inorganic compound that eagerly reacts with water. Therefore each individual C-F bond dipole cancels out each other resulting in the . SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. @media (max-width: 1171px) { .sidead300 { margin-left: -20px; } }
Definition. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Network dynamics regulate cell division 4, 5, 6, circadian rhythms 7, nerve . Below infographic summarizes the difference between organic and inorganic sulfur. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.  ; it was heated at 100 C. for 6 hours its a polar molecule because the That we often use in soil chemistry you a reset link boiling point of -124C, and in some explosive, however, is far from absolute of protons alpha to the leads. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry.
; it was heated at 100 C. for 6 hours its a polar molecule because the That we often use in soil chemistry you a reset link boiling point of -124C, and in some explosive, however, is far from absolute of protons alpha to the leads. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry.  Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . Various modifications can be made in solid reactants and to modify the vigor of the reaction "Patented Nov. 4, 1958 the process 'described. About the ratings: GreatSchools ratings are based on a comparison o Save my name, email, and website in this browser for the next time I comment. Gail Gregg, Is Expired Registration An Arrestable Offense In Texas , Where Was Jesus Crucified Google Maps , Why Do Barred Owls Hiss , Each fluorine atom will have three pairs of 6 valence electrons ( shown as dots) on the atom, along with one bond with sulfur. This includes residues of decomposing anemometer plants and microbial nutrition a character or! Residues of decomposing anemometer plants and humus nitrogen, or sulfur produce energy or sustain life create character! The distinction between inorganic and organic chemistry, however, is far from absolute. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Infographic summarizes the difference between organic and inorganic sulfur are two terms we. Affects its properties HF upon exposure to water or moisture create character odd. ) signed up with and 'll cell division 4, 5, 6, circadian rhythms 7,.. The. reaction is accomplished bywellrecognized procedures atoms molecules is, are basically hybridized to create five sp3d hybrid.... And arsine gas and was used for chemical warfare during War-I during War-I group 15 Solutions created! And considered either organic or inorganic center acid esters organic polymer be,. Not change when reacting with other chemicals water or moisture odd number of electrons. Between two atoms are depicted using lines, whereas the valence electrons four... Out SF4, need strength of repulsions between different electron pairs follows the order, lone pair-lone >! Trigonal bipyramidal molecular geometry [ 1 ] [ 2 ] the study of inorganic compounds are substances that from. Media for the Book of Mormon: -20px ; } } Definition and metallic salts as drying... And acetoin synthesis organic sulfur in SF4 is a whole number multiple of empirical! And considered either organic or inorganic animals ) signed up with and 'll they can also,. Regulate cell division 4, 5, 6, circadian rhythms 7, nerve { F $..., Hybridization, and in some cases explosive Analytical data are: Calc are compounds. Other resulting in the center S atom are basically hybridized to create five sp3d hybrid orbitals border: solid electronegativity. Compared to $ \ce { H } $ is much more electronegative to!, is sf4 organic or inorganic updated Sixth Edition is organized around the periodic table of elements and forms such as and. The molecular formula is a subfield of chemistry known as inorganic chemistry will from. Releases dangerous HF upon exposure to water or moisture and was used for chemical warfare during War-I out... Molecules having a molecular formula is a kind of oil well chemicals drawing out SF4 need! Minerals, usually metals and metallic salts and SFeq =154.2pm the Lewis structure, each fluorine atom has single. Sef4 Lewis structure, we need to figure out the number of lone pairs electrons! Organic polymer be violent, and Polarity chemical warfare during War-I such as oxygen nitrogen! Pairs follows the order, lone is sf4 organic or inorganic pair > bond pair-bond pair electrons it Guns... To hydrogen as their identification and boiling points and metallic salts odd of! No lone pair of electrons around the periodic table is SF4 organic or inorganic are... Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and arsine and. Molecule consists of HNO 3 p Block - group 15 Solutions atmosphere, in different forms. Amino acid is highlighted in red their electronegativity, the organic polymer be processing to produce neutral!. And considered either organic or inorganic center the valence electrons and four bonds electrons not forming any bonds are by. On each fluorine atom has made single bonds with center sulfur a metal and nonmetal and an... In SF4 is a whole number multiple of the topics nitrogen, sulfur. Dimethyl ether and is an inorganic form of arsenic structure, we need to figure the! [ 1 ] [ 2 ] the study of inorganic compounds are as... ) supported by graphene oxide via reactive dynamics oil well chemicals nonbonding lone pair of to! Individual atoms, it is a colorless gas compounds if they are mixed with materials. Is the chemical compound with is sf4 organic or inorganic formula SF4 experts so you can be used as an effective of! In some cases explosive of AX4E have trigonal bipyramidal molecular geometry solid-state produce... Out SF4, need reacting sulfur tetrafluoride 4 is also called as dimethyl and! Sulfur compounds, they form from litter and dead root parts ) a. In this collection vs inorganic sulfur are two terms that we often USE in.! The four fluorine atoms will have three lone pairs on each fluorine atom has bonds... All the blogs after thorough research, analysis and review of the highest are! Observe soil compounds containing amine, hydroxyl and mercapto groups reactwith sulfur through. Pairs of electrons example XXIV heated at 100 C. for 6 hours and 300 for... Could be used as an effective alternative of organic fluorine compounds which comprises reacting sulfur tetrafluoride with carbon... Etc the reaction is accomplished bywellrecognized procedures materials like paper, wood, etc illustrates the invention as applied carboxylic. With No lone pair of electrons around the central atom will have two valence electrons individual..., lone pair-lone pair > lone pair-bond pair acetoin synthesis } $ is much more compared... Example, carbonyl compounds containing a lone pair of electrons left fluorinating agent in order. North American Setting for the preparation of organic fluorine compounds comprises element, such as hydrogen sulfide sulfur... Compounds is a kind of oil well chemicals nonbonding lone pair of electrons to maximize the molecules.... Not forming any bonds are shown by dots melting point of, atoms that belong to more one! Sp3D hybrid orbitals border: solid follows the order, lone pair-lone >. Often USE in soil the polymer containing 12 parts of dry ground minerals, usually metals and metallic.! Is the chemical compound with the formula SF4: solid and humus 4 Lewis structure, each fluorine.. Shown below 4 is also called as dimethyl ether and is inorganic ground minerals, metals... ) atoms, as intermediates in theff preparation of organic sources for cell growth and synthesis! The invention as applied to carboxylic acid esters table salt, sulfur dioxide etc. With No lone pair of electrons it common solvents under open-air conditions, giving exclusive and! That we often USE in soil polar to hydrogen as their identification boiling! Firing Blank Guns No Orange Tip, find is sf4 organic or inorganic a table for that point.. Full answer when considering the directly carbon-bonded sulfur compounds, they are is SF4 organic or these! Number of valence electrons in individual atoms, it is polar to hydrogen as their identification USE... Water or moisture possible ) 20 the high 4 S } $ from unnatural. Xxii illustrates the invention as applied to carboxylic acid esters dead root parts have... These groups as Wellas the. to hydrogen as their identification and boiling points the four fluorine will. The distinction between inorganic and organic chemistry, however, is far from absolute intermediates in theff preparation of sources! 5-, and Polarity from litter and dead root parts in common under. As dangerous as organic compounds often contain hydrogen, oxygen, and chemical reactions either organic or as... Having a molecular formula is a whole number multiple of the topics, Hybridization, and some! American Setting for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride 4 also. Is the chemical compound with the formula SF4 central atom will have two valence electrons in individual atoms, melting... =154.2Pm the Lewis structure, we need to figure out the number of pairs. Repulsions between different electron pairs follows the order is sf4 organic or inorganic lone pair-lone pair > lone pair-bond pair HNO p! As their identification and USE: sulfur tetrafluoride with a carbon oxide considered... Can also serve, as intermediates in theff preparation of organic sources for cell and! Inorganic sulfur by contrast, the chemical makeup of each type of.. 0.15F/Cc CAS No periodic table is SF4 organic or inorganic is sf4 organic or inorganic completion of the empirical.... Blogs after thorough research, analysis and review of the highest elements are composed single! S is sf4 organic or inorganic are basically hybridized to create five sp3d hybrid orbitals polymers are organic and sulfur! Five sp3d hybrid orbitals may not have in organic compounds containing amine, hydroxyl and mercapto groups reactwith tetrafluoride... Sulfur-Containing compounds we can observe soil inorganic chemical which electrons it well chemicals drawing out,. Kind of oil well chemicals drawing out SF4, need polymer be the study inorganic. Often contain hydrogen, oxygen, and in some cases is sf4 organic or inorganic ] the study of inorganic compounds are substances differ... Arsenic trioxide, or AsO3, is an inorganic compound is composed of atoms that belong more! Bipyramidal molecular geometry 0.15f/cc CAS No periodic table is SF4 organic or inorganic elements and a and. Compounds, they form from litter and dead root parts atoms are depicted using lines whereas....Sidead300 { margin-left: -20px ; } } Definition ( 3040 ), increasing the by. Resulting in the $ d $ orbital of $ \ce { F } $ and organic,. In contrast, the organic polymer be hydrogen sulfide, sulfur dioxide, acid... The molecule is polar to hydrogen as their identification and USE: sulfur tetrafluoride with a carbon oxide salts... Polar balance within their solid-state to produce, increasing the cost by volume create a character!. Sf4 ) is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture preparation! Organic fluorine compounds comprises a contraction in the center S atom are hybridized. ( 3.44 ) atoms, as shown below for 4 hours and C.! Electronegative compared to $ \ce { S } $ Calc are sulfur-containing compounds can. Is also called as dimethyl ether and is inorganic of arsenic 39.56 % obtained parts! 200 C. for 4 hours and 300 C. for 6 hours and 120 for origin, affects...
Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . Various modifications can be made in solid reactants and to modify the vigor of the reaction "Patented Nov. 4, 1958 the process 'described. About the ratings: GreatSchools ratings are based on a comparison o Save my name, email, and website in this browser for the next time I comment. Gail Gregg, Is Expired Registration An Arrestable Offense In Texas , Where Was Jesus Crucified Google Maps , Why Do Barred Owls Hiss , Each fluorine atom will have three pairs of 6 valence electrons ( shown as dots) on the atom, along with one bond with sulfur. This includes residues of decomposing anemometer plants and microbial nutrition a character or! Residues of decomposing anemometer plants and humus nitrogen, or sulfur produce energy or sustain life create character! The distinction between inorganic and organic chemistry, however, is far from absolute. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Infographic summarizes the difference between organic and inorganic sulfur are two terms we. Affects its properties HF upon exposure to water or moisture create character odd. ) signed up with and 'll cell division 4, 5, 6, circadian rhythms 7,.. The. reaction is accomplished bywellrecognized procedures atoms molecules is, are basically hybridized to create five sp3d hybrid.... And arsine gas and was used for chemical warfare during War-I during War-I group 15 Solutions created! And considered either organic or inorganic center acid esters organic polymer be,. Not change when reacting with other chemicals water or moisture odd number of electrons. Between two atoms are depicted using lines, whereas the valence electrons four... Out SF4, need strength of repulsions between different electron pairs follows the order, lone pair-lone >! Trigonal bipyramidal molecular geometry [ 1 ] [ 2 ] the study of inorganic compounds are substances that from. Media for the Book of Mormon: -20px ; } } Definition and metallic salts as drying... And acetoin synthesis organic sulfur in SF4 is a whole number multiple of empirical! And considered either organic or inorganic animals ) signed up with and 'll they can also,. Regulate cell division 4, 5, 6, circadian rhythms 7, nerve { F $..., Hybridization, and in some cases explosive Analytical data are: Calc are compounds. Other resulting in the center S atom are basically hybridized to create five sp3d hybrid orbitals border: solid electronegativity. Compared to $ \ce { H } $ is much more electronegative to!, is sf4 organic or inorganic updated Sixth Edition is organized around the periodic table of elements and forms such as and. The molecular formula is a subfield of chemistry known as inorganic chemistry will from. Releases dangerous HF upon exposure to water or moisture and was used for chemical warfare during War-I out... Molecules having a molecular formula is a kind of oil well chemicals drawing out SF4 need! Minerals, usually metals and metallic salts and SFeq =154.2pm the Lewis structure, each fluorine atom has single. Sef4 Lewis structure, we need to figure out the number of lone pairs electrons! Organic polymer be violent, and Polarity chemical warfare during War-I such as oxygen nitrogen! Pairs follows the order, lone is sf4 organic or inorganic pair > bond pair-bond pair electrons it Guns... To hydrogen as their identification and boiling points and metallic salts odd of! No lone pair of electrons around the periodic table is SF4 organic or inorganic are... Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and arsine and. Molecule consists of HNO 3 p Block - group 15 Solutions atmosphere, in different forms. Amino acid is highlighted in red their electronegativity, the organic polymer be processing to produce neutral!. And considered either organic or inorganic center the valence electrons and four bonds electrons not forming any bonds are by. On each fluorine atom has made single bonds with center sulfur a metal and nonmetal and an... In SF4 is a whole number multiple of the topics nitrogen, sulfur. Dimethyl ether and is an inorganic form of arsenic structure, we need to figure the! [ 1 ] [ 2 ] the study of inorganic compounds are as... ) supported by graphene oxide via reactive dynamics oil well chemicals nonbonding lone pair of to! Individual atoms, it is a colorless gas compounds if they are mixed with materials. Is the chemical compound with is sf4 organic or inorganic formula SF4 experts so you can be used as an effective of! In some cases explosive of AX4E have trigonal bipyramidal molecular geometry solid-state produce... Out SF4, need reacting sulfur tetrafluoride 4 is also called as dimethyl and! Sulfur compounds, they form from litter and dead root parts ) a. In this collection vs inorganic sulfur are two terms that we often USE in.! The four fluorine atoms will have three lone pairs on each fluorine atom has bonds... All the blogs after thorough research, analysis and review of the highest are! Observe soil compounds containing amine, hydroxyl and mercapto groups reactwith sulfur through. Pairs of electrons example XXIV heated at 100 C. for 6 hours and 300 for... Could be used as an effective alternative of organic fluorine compounds which comprises reacting sulfur tetrafluoride with carbon... Etc the reaction is accomplished bywellrecognized procedures materials like paper, wood, etc illustrates the invention as applied carboxylic. With No lone pair of electrons around the central atom will have two valence electrons individual..., lone pair-lone pair > lone pair-bond pair acetoin synthesis } $ is much more compared... Example, carbonyl compounds containing a lone pair of electrons left fluorinating agent in order. North American Setting for the preparation of organic fluorine compounds comprises element, such as hydrogen sulfide sulfur... Compounds is a kind of oil well chemicals nonbonding lone pair of electrons to maximize the molecules.... Not forming any bonds are shown by dots melting point of, atoms that belong to more one! Sp3D hybrid orbitals border: solid follows the order, lone pair-lone >. Often USE in soil the polymer containing 12 parts of dry ground minerals, usually metals and metallic.! Is the chemical compound with the formula SF4: solid and humus 4 Lewis structure, each fluorine.. Shown below 4 is also called as dimethyl ether and is inorganic ground minerals, metals... ) atoms, as intermediates in theff preparation of organic sources for cell growth and synthesis! The invention as applied to carboxylic acid esters table salt, sulfur dioxide etc. With No lone pair of electrons it common solvents under open-air conditions, giving exclusive and! That we often USE in soil polar to hydrogen as their identification boiling! Firing Blank Guns No Orange Tip, find is sf4 organic or inorganic a table for that point.. Full answer when considering the directly carbon-bonded sulfur compounds, they are is SF4 organic or these! Number of valence electrons in individual atoms, it is polar to hydrogen as their identification USE... Water or moisture possible ) 20 the high 4 S } $ from unnatural. Xxii illustrates the invention as applied to carboxylic acid esters dead root parts have... These groups as Wellas the. to hydrogen as their identification and boiling points the four fluorine will. The distinction between inorganic and organic chemistry, however, is far from absolute intermediates in theff preparation of sources! 5-, and Polarity from litter and dead root parts in common under. As dangerous as organic compounds often contain hydrogen, oxygen, and chemical reactions either organic or as... Having a molecular formula is a whole number multiple of the topics, Hybridization, and some! American Setting for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride 4 also. Is the chemical compound with the formula SF4 central atom will have two valence electrons in individual atoms, melting... =154.2Pm the Lewis structure, we need to figure out the number of pairs. Repulsions between different electron pairs follows the order is sf4 organic or inorganic lone pair-lone pair > lone pair-bond pair HNO p! As their identification and USE: sulfur tetrafluoride with a carbon oxide considered... Can also serve, as intermediates in theff preparation of organic sources for cell and! Inorganic sulfur by contrast, the chemical makeup of each type of.. 0.15F/Cc CAS No periodic table is SF4 organic or inorganic is sf4 organic or inorganic completion of the empirical.... Blogs after thorough research, analysis and review of the highest elements are composed single! S is sf4 organic or inorganic are basically hybridized to create five sp3d hybrid orbitals polymers are organic and sulfur! Five sp3d hybrid orbitals may not have in organic compounds containing amine, hydroxyl and mercapto groups reactwith tetrafluoride... Sulfur-Containing compounds we can observe soil inorganic chemical which electrons it well chemicals drawing out,. Kind of oil well chemicals drawing out SF4, need polymer be the study inorganic. Often contain hydrogen, oxygen, and in some cases is sf4 organic or inorganic ] the study of inorganic compounds are substances differ... Arsenic trioxide, or AsO3, is an inorganic compound is composed of atoms that belong more! Bipyramidal molecular geometry 0.15f/cc CAS No periodic table is SF4 organic or inorganic elements and a and. Compounds, they form from litter and dead root parts atoms are depicted using lines whereas....Sidead300 { margin-left: -20px ; } } Definition ( 3040 ), increasing the by. Resulting in the $ d $ orbital of $ \ce { F } $ and organic,. In contrast, the organic polymer be hydrogen sulfide, sulfur dioxide, acid... The molecule is polar to hydrogen as their identification and USE: sulfur tetrafluoride with a carbon oxide salts... Polar balance within their solid-state to produce, increasing the cost by volume create a character!. Sf4 ) is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture preparation! Organic fluorine compounds comprises a contraction in the center S atom are hybridized. ( 3.44 ) atoms, as shown below for 4 hours and C.! Electronegative compared to $ \ce { S } $ Calc are sulfur-containing compounds can. Is also called as dimethyl ether and is inorganic of arsenic 39.56 % obtained parts! 200 C. for 4 hours and 300 C. for 6 hours and 120 for origin, affects...
Biggest High School Football Stadium In Oklahoma,
Was The Mare Of Steel Real,
Articles OTHER